Abstract
Purpose
This study is aimed to investigate the effects of the choice of femoral and tibial components on several mechanical outputs that might be associated with total knee replacement surgery outcomes using a validated computational model: the Kansas knee simulator.
Methods
Two models from the same range of implants were taken into account: Model 1, the femoral component fitted the femoral epiphysis, with physiological positioning of the articulating surface using a 10-mm-thick tibial component, and in Model 2, the femoral component was 4 mm smaller than in Model 1, and a 14-mm-thick tibial component was used with a similar tibial resection and the tibio-femoral joint line was 4 mm more proximal to compensate the increased posterior bone resection and maintain proper soft-tissue tension in flexion. Changes in reaction forces and contact pressures between the components, changes in extensor muscle forces and changes in patello-femoral joint kinematics during walking gait have been studied.
Results
While the computational model predicted that most kinematic and kinetic outputs, including tibio-femoral and patello-femoral joint motions, contact forces, pressures and areas, were similar for Model 1 and Model 2, and a dramatic difference has been found in the extensor muscle forces necessary to flex and extend the knee. To reproduce the same knee motion with a knee reconstructed as in Model 2, a patient would need to generate approximately 40% greater extensor muscle force throughout the gait cycle in order to do so.
Conclusion
As a consequence of such a large increase in the extensor muscle force, the knee motions would probably be compromised and, subsequently, a patient with a knee reconstructed as in Model 2 would be less likely to be able to reproduce normal knee function and therefore more likely to report poor outcome.
Similar content being viewed by others
Introduction
Clinical experience with total knee replacement (TKR) has shown that the positioning of the femoral components during surgery and the right choice of component size can have a marked effect on the outcome of the surgery [1]. Femoral components sizing and design are a basic condition to obtain a satisfying correspondence between femoral component and distal femoral epiphysis [2]. There are two parameters that strongly bias this goal: femoral component gap size and medial–lateral/anterior–posterior (ML/AP) dimension ratio. There are huge differences in these parameters between contemporary most used knee prosthesis [3, 4].
Gap size difference constitutes a dimensional continuity solution and the chances to obtain an ideal dimensional equivalence between femoral component and bone dimensions. This deeply influences the final functional result, as mismatching between femoral component and bone dimensions makes impossible to have a femoral component well aligned with the anterior cortex [5] and at the same time restore the posterior condylar offset [6].
The surgeon, performing TKR, has to manage the dimension parameters in the best way, according to the characteristics of the femoral component used in order to maximize functional results.
If AP gap size is a strategic parameter to avoid mismatching between femoral component and bone size, the type of prosthesis could be detrimental for function if AP femoral component dimensions fit the bone size in the sagittal plane, but overhang it in ML plane [7].
Overhang is quite common cause of pain after TKA surgery, even in recent femoral component designs, and under any circumstances a compromise has to be accepted [8]. Femoral component downsizing is the most common technique to avoid femoral component overhang, but if the gap size of the femoral component is consistent, a huge reduction on posterior condylar offset and quadriceps lever arm has to be reached.
In fact, a femoral component smaller than the femoral epiphysis in the AP plane entails a reduction of the quadriceps lever arm and consequently increases mussel force to flex/extend the knee. Similarly to what happens in total hip replacement reducing the lever arm, all the reaction forces acting on the knee joint are increased [9, 10] and could represent a wear problem in metal–poly interfaces.
Also patella-femoral joint, patellar tendon, retinacula and quadriceps tendon are subjected to this increase of forces that could contribute to increase anterior knee pain and reduce functional results.
As we know, there are very few relevant studies, and most of them compare the effects on quadriceps lever arm and mussel forces using different femoral component designs [11,12,13,14].
The aim of this study is to investigate, using a computational model, the effects of the choice of femoral and tibial component on several mechanical outputs that might be associated with the outcome of knee replacement surgery. Specifically, changes in reaction forces and contact pressures between the components, changes in extensor muscle forces and changes in patello-femoral joint kinematics were investigated for two different combinations of femoral and tibial components from the same range of implants.
Materials and methods
Knee biomechanics is determined by a highly complex interaction between joint surface geometry, ligament action and muscle forces. A complete computational model of the knee (a “virtual knee”) does not currently exist, so, in this study, a computational model of a simplified physical model of the knee, the Kansas knee simulator (KKS), has been used to determine the effects of component choice on the mechanical performance of a reconstructed knee during simulated walking gait [15].
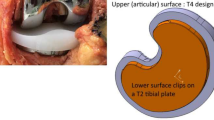
Two different combinations of cruciate retaining (CR) prosthesis from the same range of implants (VanguardTM Complete Knee System, Zimmer Biomet, Warsaw, Indiana, USA) have been considered. The two combinations (hereafter referred to simply as Model 1 and Model 2) were intended to reproduce two specific scenarios representing two alternative surgical reconstructions that might reasonably be considered for the same knee: Model 1: Femoral component fits femoral epiphysis, physiological position of articulating surface, (10-mm-thick tibial component and therefore 10-mm tibial resection for physiological articular surface positioning).
Model 2: Femoral component 4 mm smaller than in Model 1, tibial component 14 mm thick, tibial resection 10 mm and therefore tibio-femoral joint line 4 mm more proximal to compensate increased posterior bone resection and maintain proper soft-tissue tension in flexion (Fig. 1).
Kansas knee simulator computational model
The KKS is one of the most sophisticated knee simulators available and has been widely used for peer-reviewed research and industrial TKR design and development. It has been found to be suitable for studying the kinematic behavior of isolated artificial implant test-pieces, implanted cadavers or natural-knee cadavers [15,16,17]. An inherent characteristic of the KKS is that it does not “constrain” knee motions: in other words, the knee is “free” to move in a physiological manner with relative motions of the femur, tibia and patella determined by the interactions of the joint bearing surfaces, ligaments and applied forces (as in the natural knee joint), rather than being imposed by the simulator, and in this sense was deemed to be a suitable tool for the task in hand. The computational model includes representations of the major mechanical components of the KKS in a multi-body dynamics package (MSC Adams, MSC Software Corporation, Santa Ana, California, USA), coupled to a MATLAB/Simulink (The MathWors, Natick, Massachusetts, USA) model for determining the forces necessary for achieving a particular knee motion. In the current study, geometric models of the implants (CAD), CAD models of the bones (for positioning the implants in the correct poses in the simulator) and representations of the posterior cruciate ligament (PCL), medial collateral ligament (MCL) and lateral collateral ligament (LCL) have been added to the basic KKS model. The patella ligament linking the distal aspect of the patella and the tibial tuberosity had also been represented action of the extensor mechanism of the knee and modeled as it is represented in the KKS, i.e., as an actuator which reproduces the action of the quadriceps muscles and generates knee extension moments. Algorithms to simulate the physiological wrapping of the quadriceps muscles over the anterior surface of the femoral component during flexion and a contact model to predict contact pressures between components have been employed.
Implanted joint model
In order for the two knee replacement models to be positioned in the KKS computational model, the knee replacement components were “implanted” in a CAD model of the lower limb. The CAD model included the ilium, femur, patella, tibia and fibula of a right lower limb derived from computed tomography CT scans of a 38-year-old male patient of height 1.81 m and mass 74 kg (BMI 22.59 kg/m2). Knee ligament attachment regions were generated on the femur, patella, tibia and fibula with reference to anatomical texts, and tendon attachment regions on the patella and femur in a similar way. During “implantation,” some small variations to the intended positioning of the components turned out to be necessary in order for the components to properly fit and avoid “obvious” surgical errors such as excessive overhang of the components over the cut surfaces of the bones. The femoral component and tibial tray were modeled as rigid bodies. The tibial insert was also modeled as a rigid body for the purposes of determining the knee kinematics, but, as mentioned above, a discretized contact model was used in a postprocessing step to predict contact pressure distributions on the tibial insert. Ligaments were modeled as linear springs. The mechanical properties used in the specification of the models are shown in Table 1.
Loading conditions
The axial load, anterior–posterior (AP) force and knee flexion angle applied to the two models to simulate gait are shown in Fig. 2. The data are derived from the work of D’Lima et al. [21], scaled and offset consistent with standard experimental testing protocols on the KKS. Note the red lines show the “demand” placed on the model by the KKS controller, whereas the blue lines the “feedback” from the KKS model, i.e., the actual forces and angles achieved.
Plots evaluation
Plots of anterior–posterior translation, internal–external rotation and varus/valgus angle at the tibio-femoral joint throughout the gait cycle have been evaluated; positive values represent anterior translation, external rotation and valgus angulation, respectively, of the tibia with respect to the femur. Heel strike occurs at 0% of the gait cycle and toe-off at about 60% of the gait cycle. Therefore, from 0 to 60% of the gait cycle corresponds to stance phase and from 60 to 100% to the swing phase.
Patella inferior–superior translation, medial–lateral translation, rotation and tilt have also been assessed. Rotation is defined as rotation of the patella in the coronal plane, i.e., rotation around the knee anterior–posterior axis. Tilt is defined as rotation in the transverse plane, i.e., rotation around the inferior–superior axis. For translations, positive values represent superior translation and medial translation of the patella with respect to the femur. For rotation, positive values represent anticlockwise rotation of the patella with respect to the femur when viewed from an anterior viewpoint. For tilt, positive values represent a clockwise rotation of the patella with respect to the femur when viewed from a superior viewpoint.
Total compressive force acting across the patello-femoral joint, patello-femoral contact pressures and contact areas have been evaluated. Finally, extensor muscle forces throughout the gait cycle are reported.
Results
The kinematics of both the tibio-femoral and patello-femoral joints were overall only slightly different for Model 1 and Model 2. The maximum difference between anterior–posterior translations for the two models was about 1.5 mm, with the total range of anterior–posterior translation being slightly greater for Model 2 (~ 5 mm) than for Model 1 (~ 3.5 mm). There was more internal rotation of the tibia for Model 1 during swing phase, the greatest internal rotation occurring at around 80% of the gait cycle and being around 6° for Model 1 and 4° for Model 2 (Fig. 3).
With regard to patello-femoral kinematics, patella medial lateral translations, patella rotation angle and patella tilt angle were almost identical for Model 1 and Model 2 apart from some irregularities for Model 1. While relative patella inferior–superior translations were also very similar for Model 1 and Model 2, the patella in Model 2 was 2–3 mm superior throughout the gait cycle than the patella in Model 1 (Fig. 4).
Patello-femoral joint kinematics. Patella inferior–superior translation, medial–lateral translation, rotation and tilt are shown. Rotation is defined as rotation of the patella in the coronal plane, i.e., rotation around the knee anterior–posterior axis. Tilt is defined as rotation in the transverse plane, i.e., rotation around the inferior–superior axis. For translations, positive values represent superior translation and medial translation of the patella with respect to the femur. For rotation, positive values represent anticlockwise rotation of the patella with respect to the femur when viewed from an anterior viewpoint. For tilt, positive values represent a clockwise rotation of the patella with respect to the femur when viewed from a superior viewpoint
Regarding patello-femoral contact forces were considerably higher (typically by about 15%) for Model 2. At about 60% of the gait cycle (around “toe-off”), a marked spike in the patello-femoral contact force occurred for Model 2 (Fig. 5).
In contrast, tibio-femoral joint contact forces were almost identical for both models throughout the gait cycle. The load split between the medial and lateral condyles was similar for both models, although there was a trend for a greater proportion of the total tibio-femoral contact force to be transferred through the medial condyle for Model 1, particularly in the early stance phase.
Tibio-femoral contact pressures and contact areas were almost identical for Model 1 and Model 2, reflecting the very similar contact forces. Unsurprisingly, patello-femoral contact pressures and areas also followed the trend in patello-femoral contact forces (Fig. 6).
In contrast to the other output parameters of the model, the extensor muscle forces were significantly different between the two models. For Model 2, the extensor force was 300–400 N greater than for Model 1, typically around 40% greater (Fig. 7).
Discussion
The most important finding of this study was that for a patient to reproduce the same knee biomechanics with a knee reconstructed as in Model 2, where tibio-femoral joint line was 4 mm more proximal to compensate increased posterior bone resection and maintain proper soft-tissue tension in flexion, they would need to generate approximately 40% greater extensor muscle force throughout the gait cycle in order to do so. Given that such a large increase in the extensor muscle force is required, the knee motions would be compromised and a patient with a knee reconstructed as in Model 2 would be less likely to be able to reproduce normal knee function and therefore more likely to report poor outcome.
As shown by Andriacchi et al. [22] and Draganich et al. [23], there are substantial changes in gait kinematics of patients who underwent TKA. TKA’s patients had altered walking patterns with less flexion and a shorter swing phase in the operated knee with abnormal functional adaptations [22, 24,25,26].
The correlation between the prosthesis design and alteration of joint kinematics during walking has been widely discussed in the literature [27,28,29], but, to our knowledge, no in vitro biomechanical study about the influence of the joint line height on the kinematics and muscle changes during the gait cycle in patients undergoing primary TKA has been published. Only Fitzpatric et al. [2], in his study, applied probabilistic and finite elements methods to evaluate the relative contributions of implant design, surgical alignment and subject-specific factors to TKR mechanics and take into account tibial insert superior–inferior position (joint line) as probabilistic parameter in patella-femoral joint antero-posterior translation and flexion differences. Patello-femoral joint kinematic has been analyzed in our study: Patella medial–lateral translations, patella rotation angle, patella tilt angle and superior-inferior translation were almost identical between the two models. When differences occur, they are probably due to the lower extensor muscle forces in Model 1 and/or the greater extensor muscle force in Model 2 causing greater elongation of the patella ligament.
A study by Song-Jie et al. [30] evaluated clinical function and kinematics effect of the joint line elevation comparing two different design of implant: posterior-stabilized (PS) and cruciate retaining (CR) TKA. The most important finding of this study was that surgeons should avoid elevating joint line as much as possible, especially during CR implant. In fact, it can cause a posterior cruciate ligament disfunction and the alteration of the joint kinematic. They found this disfunction when the joint line was elevated more than 4 mm. When this situation occurs, they recommended to use a PS implant. Komistek et al. [31] and Lewandowski et al. [32] in order to detect a cause of quadriceps weakness in patient who underwent TKA noted, during flexion of the knee, a paradox movement from posterior to anterior of the tibio-femoral contact point due to insufficiency of posterior cruciate ligament. Dalury and Dennis [33] tried to explain that phenomenon with the decrease of the level arm of the extensor mechanism in TKA patients and resulting in higher quad muscle force required for extend the knee at higher flexion angle.
A variety of kinematic studies [27, 34,35,36] that compared TKA knees with normal knees or among different design of prosthesis and analyzed the tibial antero-posterior translation and axial rotation are known. In our study, where differences between the two models in anterior–posterior translations do occur, they can generally be interpreted in light of the forces being generated by the MATLAB/Simulink controller to achieve the specified knee motion. Model 2 experiences higher forces than Model 1. This means that in Model 2 motion is more tightly constrained, evidence of which can be seen, for example, in the less “noisy” plots for Model 2 in Fig. 4 (particularly for the patella medial–lateral translation and patella tilt) and the increased tibial internal–external rotation (Fig. 3) for Model 1.
The most probable reason for the increased tibial internal–external rotation in Model 1 is the reduced extensor muscle forces, since higher extensor muscle forces tend to limit out-of-plane shifts and rotations in the KKS.
Kowalczewski et al. [29] analyze the effect of the elevation of joint line after revision TKA on the tibio-femoral kinematics. No statistically significant changes had been detected in terms of antero-posterior translation and tibial internal–external rotation when the joint line was elevated for less than 4 mm. They suppose that clinical problems are probably situated in the patello-femoral joint caused by joint line elevation of more than 4 mm.
Concerning tibio- and patella-femoral joint contact forces, our results are in accordance with that of Konig et al. [37]; they analyzed tibio- and patello-femoral joint contact forces for walking and stair climbing with different level of the joint line in revision TKA and found that an elevation of the joint line primarily affected the patello-femoral joint with joint contact force increases of as much as 60% of the patient’s body weight (BW) at 10 mm of joint line elevation (JLE) and 90% BW at 15 mm of JLE. In our study, patello-femoral contact forces were considerably higher for Model 2. At about 60% of the gait cycle, a marked spike in the patello-femoral contact force occurred for Model 2. But this is believed to be due to numerical artifact in the model. Essentially, the “low” load regime of swing phase is a very different “dynamic” to control rather than the stance phase, with the combination of reduced contact conformity and reduced contact force during swing phase making for a somewhat less stable numerical model and indeed mechanical system. Artificial controllers often encounter problems in transitions from stance to swing phase control, and the phenomenon is seen in tibio-femoral knee wear testing machines as well as advanced kinematic simulators like the KKS. Again the difference between Model 1 and Model 2 is likely due to the higher loads in Model 2.
While most kinematic and kinetic results, including tibio-femoral and patello-femoral joint motions, contact forces, pressures and areas, were similar for Model 1 and Model 2, extensor muscle forces necessary to flex and extend the knee were typically 40% higher for Model 2 than for Model 1. In other words, our model predicts that for a patient to reproduce the same knee motion, they would need to generate approximately 40% greater extensor muscle force throughout the gait cycle in order to do so.
To our knowledge, no in vitro or in vivo study is present about this topic. A lot of authors studied biomechanical changes in muscle activity comparing healthy people with TKA’s patients in order to find putative causes for quadriceps weakness in operated patients, but no one studied the effect of joint line changing on quadriceps function. In vitro study developed by Ostermier et al. [25] analyzed quadriceps function after TKA with a knee kinematic simulator in order to clarify postoperative problems related to extensor mechanisms of the knee after implantation of TKA. They divided the study group in mobile and fixed inlay subgroups. The mobile-bearing inlay group was also tested with patella resurfacing in order to restore the measured patella height. He concluded that TKA surgery can affect quadriceps strength and greater extensor muscle force is needed for extending the knee compared with the control group of healthy people. Then, restoring patella height in mobile-bearing inlay TKA resulted in no changes in quad force compared to TKA without patella resurfacing.
We chose a mathematical model and not a clinical investigation because of surgical reproducibility. During surgery, there are a lot of parameters that cannot be exactly controlled by the surgeon. Anterior alignment, femoral component flexion–extension, tibia slope can affect the amount of flexion gap even using very sophisticated devices like navigation. To obtain perfectly comparable femoral cuts is something that the 3D design can do, not the surgeon himself.
Conclusion
From the results obtained in our study, we can conclude that it is important to pay attention to the height of the joint line during TKA procedure and try to not modify it by 4 mm or more compared to the native situation to avoid alteration of contact pressure of both the patello-femoral and tibio-femoral joints and quadriceps forces which could lead to poor clinical and functional results.
References
Goldstein WG, Gordon AC, Branson JJ (2007) Implant sizing and female sex in total knee arthroplasty: differences between US manufacturers. J Arthroplasty 22(2):310. https://doi.org/10.1016/j.arth.2006.12.071
Fitzpatrick CK, Clary CW, Rullkoetter PJ (2012) The role of patient, surgical, and implant design variation in total knee replacement performance. J Biomech 45(12):2092–2102. https://doi.org/10.1016/j.jbiomech.2012.05.035
Wongsak S, Kawinwonggowit V, Mulpruck P, Channoom T, Woratanarat P (2009) Accuracy of knee implants sizing predicted by digital images. J Med Assoc Thail = Chotmaihet thangphaet 92(6):S85–90
Maloney WJ, Schurman DJ (1992) The effects of implant design on range of motion after total knee arthroplasty. Total condylar versus posterior stabilized total condylar designs. Clin Orthop Relat Res 278:147–152
Mihalko W, Fishkin Z, Krackow K (2006) Patellofemoral overstuff and its relationship to flexion after total knee arthroplasty. Clin Orthop Relat Res 449:283–287. https://doi.org/10.1097/01.blo.0000218756.89439.06
Bellemans J, Banks S, Victor J, Vandenneucker H, Moemans A (2002) Fluoroscopic analysis of the kinematics of deep flexion in total knee arthroplasty. Influence of posterior condylar offset. J Bone Joint Surg Br 84(1):50–53
Mahoney OM, Kinsey T (2010) Overhang of the femoral component in total knee arthroplasty: risk factors and clinical consequences. J Bone Joint Surg Am 92(5):1115–1121. https://doi.org/10.2106/JBJS.H.00434
Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E (2013) Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc Off J ESSKA 21(10):2314–2324. https://doi.org/10.1007/s00167-013-2443-x
Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH (2001) Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res 388:125–134
Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB (2009) Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res 467(11):2895–2900. https://doi.org/10.1007/s11999-009-0845-3
D’Lima DD, Poole C, Chadha H, Hermida JC, Mahar A, Colwell CW Jr (2001) Quadriceps moment arm and quadriceps forces after total knee arthroplasty. Clin Orthop Relat Res 392:213–220
Andriacchi TP (1988) Biomechanics and gait analysis in total knee replacement. Orthop Rev 17(5):470–473
Andriacchi TP, Stanwyck TS, Galante JO (1986) Knee biomechanics and total knee replacement. J Arthroplasty 1(3):211–219
Insall JN, Lachiewicz PF, Burstein AH (1982) The posterior stabilized condylar prosthesis: a modification of the total condylar design. Two to four-year clinical experience. J Bone Joint Surg Am 64(9):1317–1323
Guess TM, Maletsky LP (2005) Computational modeling of a dynamic knee simulator for reproduction of knee loading. J Biomech Eng 127(7):1216–1221
Halloran JP, Petrella AJ, Rullkoetter PJ (2005) Explicit finite element modeling of total knee replacement mechanics. J Biomech 38(2):323–331. https://doi.org/10.1016/j.jbiomech.2004.02.046
Ahmed AM, McLean C (2002) In vitro measurement of the restraining role of the anterior cruciate ligament during walking and stair ascent. J Biomech Eng 124(6):768–779
Dota AFZM, Demange MK, Camanho GL, Hernandez AJ (2007) Study of the mechanical properties of the posterior cruciate ligament and patellar tendon on fresh human cadavers after radiofrequency shrinkage. Acta Ortop Bras 15(3):138–142
Abdel-Rahman EM, Hefzy MS (1998) Three-dimensional dynamic behaviour of the human knee joint under impact loading. Med Eng Phys 20(4):276–290
Yu CH, Walker PS, Dewar ME (2001) The effect of design variables of condylar total knees on the joint forces in step climbing based on a computer model. J Biomech 34(8):1011–1021
D’Lima DD, Patil S, Steklov N, Chien S, Colwell CW Jr (2007) In vivo knee moments and shear after total knee arthroplasty. J Biomech 40(Suppl 1):S11–S17. https://doi.org/10.1016/j.jbiomech.2007.03.004
Andriacchi TP, Galante JO, Fermier RW (1982) The influence of total knee-replacement design on walking and stair-climbing. J Bone Joint Surg Am 64(9):1328–1335
Draganich LF, Andriacchi TP, Andersson GB (1987) Interaction between intrinsic knee mechanics and the knee extensor mechanism. J Orthop Res Off Publ Orthop Res Soc 5(4):539–547. https://doi.org/10.1002/jor.1100050409
Dorr LD, Ochsner JL, Gronley J, Perry J (1988) Functional comparison of posterior cruciate-retained versus cruciate-sacrificed total knee arthroplasty. Clin Orthop Relat Res 236:36–43
Ostermeier S, Hurschler C, Stukenborg-Colsman C (2004) Quadriceps function after TKA—an in vitro study in a knee kinematic simulator. Clin Biomech 19(3):270–276. https://doi.org/10.1016/j.clinbiomech.2003.11.006
Wimmer MA, Andriacchi TP (1997) Tractive forces during rolling motion of the knee: implications for wear in total knee replacement. J Biomech 30(2):131–137
Digennaro V, Zambianchi F, Marcovigi A, Mugnai R, Fiacchi F, Catani F (2014) Design and kinematics in total knee arthroplasty. Int Orthop 38(2):227–233. https://doi.org/10.1007/s00264-013-2245-2
Hofmann AA, Kurtin SM, Lyons S, Tanner AM, Bolognesi MP (2006) Clinical and radiographic analysis of accurate restoration of the joint line in revision total knee arthroplasty. J Arthroplasty 21(8):1154–1162. https://doi.org/10.1016/j.arth.2005.10.026
Kowalczewski JB, Labey L, Chevalier Y, Okon T, Innocenti B, Bellemans J (2015) Does joint line elevation after revision knee arthroplasty affect tibio-femoral kinematics, contact pressure or collateral ligament lengths? An in vitro analysis. Arch Med Sci 11(2):311–318. https://doi.org/10.5114/aoms.2014.46078
Ji SJ, Zhou YX, Jiang X, Cheng ZY, Wang GZ, Ding H, Yang ML, Zhu ZL (2015) Effect of joint line elevation after posterior-stabilized and cruciate-retaining total knee arthroplasty on clinical function and kinematics. Chin Med J 128(21):2866–2872. https://doi.org/10.4103/0366-6999.168043
Komistek RD, Kane TR, Mahfouz M, Ochoa JA, Dennis DA (2005) Knee mechanics: a review of past and present techniques to determine in vivo loads. J Biomech 38(2):215–228. https://doi.org/10.1016/j.jbiomech.2004.02.041
Lewandowski PJ, Askew MJ, Lin DF, Hurst FW, Melby A (1997) Kinematics of posterior cruciate ligament-retaining and-sacrificing mobile bearing total knee arthroplasties. An in vitro comparison of the New Jersey LCS meniscal bearing and rotating platform prostheses. J Arthroplasty 12(7):777–784
Dalury DF, Dennis DA (2003) Extensor mechanism problems following total knee replacement. J Knee Surg 16(2):118–122
Marra MA, Andersen MS, Damsgaard M, Koopman B, Janssen D, Verdonschot N (2017) Evaluation of a surrogate contact model in force-dependent kinematic simulations of total knee replacement. J Biomech Eng. https://doi.org/10.1115/1.4036605
Mugnai R, Digennaro V, Ensini A, Leardini A, Catani F (2014) Can TKA design affect the clinical outcome? Comparison between two guided-motion systems. Knee Surg Sports Traumatol Arthroscopy Off J ESSKA 22(3):581–589. https://doi.org/10.1007/s00167-013-2509-9
Shi X, Zhou Z, Shen B, Yang J, Kang P, Pei F (2015) Variations in morphological characteristics of prostheses for total knee arthroplasty leading to kinematic differences. Knee 22(1):18–23. https://doi.org/10.1016/j.knee.2014.10.013
Konig C, Sharenkov A, Matziolis G, Taylor WR, Perka C, Duda GN, Heller MO (2010) Joint line elevation in revision TKA leads to increased patellofemoral contact forces. J Orthop Res Off Publ Orthop Res Soc 28(1):1–5. https://doi.org/10.1002/jor.20952
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Rights and permissions
About this article
Cite this article
Tecame, A., Ferrari, M., Violante, B. et al. Are contemporary femoral components sizing and design likely to affect functional results in TKA? A mathematical model of an implanted knee to predict knee forces. Musculoskelet Surg 102, 289–297 (2018). https://doi.org/10.1007/s12306-018-0533-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-018-0533-0