Abstract
In this paper, we remind readers of several ICH guideline documents such as ICH Q3A, Q3B, Q3C, Q3D, Q6A, Q6B, M7, and ICH S9 which are related to the drug substance and drug product impurity limit setting. In particular, ICH Q6A clearly states that “specifications should focus on those characteristics found to be useful in ensuring the safety and efficacy of the drug substance and drug product”; however, recent negotiations between health authority and applicants (company) related to proposed marketing applications show that on a global level, batch experience, even when limited, plays an overwhelming role in developing impurity acceptance criteria rather than clinical relevance. The drawback of such practice and the great need to establish patient centric specifications (PCS) are highlighted. Secondly, this paper proposes approaches on how to establish patient centric criteria for drug substance and drug product impurity limits based on the principles outlined in ICH guideline documents and scientific literature. Three case studies are presented to illustrate the challenges in establishing PCS and the divergence of regulatory acceptance to such specifications. We propose some approaches that can be considered for specification setting based on clinical relevance in the drug development, registration and post-approval phases of a product life-cycle. Lastly, we give thoughts on the future perspective of this movement and offer recommendations to foster discussions between regulatory agencies and pharmaceutical industry on getting medicinal products that are safe and effective to the patient sooner to meet unmet medical needs without supply interruption concerns.
Similar content being viewed by others
Introduction
The following White Paper describes the International Society for Pharmaceutical Engineering (ISPE)-sponsored Patient-Centric Specification (PCS) Working Group’s current thinking on this topic as applied to drug substance and drug product impurities. The term “patient-centric specification” builds on existing industry guidance and utilizes the science and knowledge generated through the development and life-cycle of a pharmaceutical product. This white paper is intended to further stimulate and support ongoing discussions to ensure that the quality, safety, and efficacy remain focused on patients’ needs and the access to medicines is improved through a reliable, robust supply chain. Through this approach to the adoption of patient centric specifications as a standard for pharmaceutical manufacturing, industry, and regulators can achieve the simultaneous attainment of two worthy goals: providing a reliable supply of safe and efficacious products to patients while reducing manufacturing costs. This approach aligns well with the vision of the USA’s twenty-first Century Cures Act (Cures Act), which was signed into law on December 13, 2016, and is designed to help accelerate medical product development and bring new innovations and advances to patients who need them faster and more efficiently [1]. The US FDA has developed and submitted to Congress (June 9, 2017) a work plan which describes activities to achieve the Cures Act requirements. One of the key components is to include the patient’s voice in drug development and review [2].
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) finalized two critically important guidelines in 1999. ICH Q6B, “Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biologic Products” was completed in March, with ICH Q6A, “Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances” being completed in October that same year [3, 4]. Both provide extensive guidance on establishing specifications. While the guidances are nearly two decades old, the prevailing principles are as relevant today as they were then; “establishment of a single set of global specifications for new drug substances and new drug products” [3]. Like all ICH guidances, the primary objectives of ICH Q6A and Q6B are to bring harmonization across the member regions when setting specifications for pharmaceutical product registration and the maintenance of such registrations; however, given time and experience, it is not unexpected to see some level of divergence of interpretations and renewed thought processes on this complex topic. In addition, ICH itself has evolved with the newer quality guidelines adopting a patient-focused, science, and risk-based approach. It is therefore an appropriate time to re-examine the Q6 guidelines against the stated intentions and the current ICH quality vision of “A harmonized pharmaceutical quality system applicable throughout the life cycle of the product, emphasizing an integrated approach to risk management and science” [5].
ICH Q6A defines “specification as a list of tests, references to analytical procedures, and appropriate acceptance criteria, which are numerical limits, ranges or other criteria for the tests described. It establishes the set of criteria to which a drug substance or drug product should conform to be considered acceptable for its intended use”. The guideline goes on to state specifications “should focus on those characteristics found to be useful in ensuring the safety and efficacy of the drug substance and drug product”. It is unfortunate that this fundamental basis of specifications with a focus on safety and efficacy is sometimes obscured by regional regulatory agencies’ expectations that batch manufacturing history is paramount to setting specifications at the point of registration. Those expectations should not come as a surprise since, for example, ICH Q6B states “Further, the acceptance criteria for impurities should be based on data obtained from lots used in preclinical and clinical studies and manufacturing consistency lots.” This tension between batch data and safety data is more disruptive when there is very limited batch data available. It is, however, unsurprising that this conflict exists when Q6A specifically directs that the acceptance criterion for a drug substance (DS) impurity be set based on the mean+upper confidence level seen in “relevant” batches. Such an approach has been referred to as quality by testing and this conflict was recognized when the ICH quality experts agreed their desired state (mission statement) in support of the ICH quality vision that was established in 2003 [5]. This desired state incorporated the fundamental elements described in the FDA’s guidance on Process Analytical Technology [6].
-
Product quality and performance achieved and assured by design of effective and efficient manufacturing processes
-
Product specifications based on mechanistic understanding of how formulation and process factors impact product performance
-
An ability to effect continuous improvement and continuous “real time” assurance of quality
-
Regulatory policies tailored to recognize the level of scientific knowledge supporting product applications, process validation, and process capability
-
Risk-based regulatory scrutiny related to the level of scientific understanding of how formulation and manufacturing process factors affect product quality and performance, and the capability of process control strategies to prevent or mitigate risk of producing a poor quality product
-
Barriers to continuous improvement reduced or removed
-
Improved manufacturing efficiency
-
Sustained or improved product quality
-
Specifications based on parameters that truly impact product quality
-
Common understanding and language on risk
-
Both, industry and regulatory authorities focus on areas of greatest risk and understanding of residual risks
This combination of quality vision and desired state has guided the development of the new era of science and risk-based guidelines that started with the trio of ICH Q8, Q9, and Q10 with their focus on the needs of the patient and “Quality by Design” [7,8,9,10].
Patient Centric Specifications
A patient centric specification is one that focuses on patient needs (product safety and efficacy) and improves access to medicines through reliable, robust supply chains. A number of published papers and industry/regulatory forums have opened the door to discussing patient centric specification (PCS), which was also referred to as clinically relevant specifications (CRS) in the literatures. To date, there is no harmonized definition of PCS or CRS, and the thinking has continued to evolve. In 2012, FDA suggested “CRS are those specifications that take into consideration the clinical impact of variations in the critical quality attributes (CQA) and process parameters assuring a consistent safety and efficacy profile” [11]. At the 2014 PQRI conference on product quality, FDA offered the definition “Test methods and acceptance criteria that identify and reject / accept drug product batches that are likely to perform inadequately / adequately in the indicated patient population(s) [12].” In their 2015 white paper, FDA’s Office of Pharmaceutical Quality defined CRS as “a set of criteria and acceptance ranges to which drug products should conform in order to deliver the therapeutic benefit indicated in the label” [13]. CRS acceptance criteria are established based on clinical relevance when known, instead of process capability or manufacturing process control. The FDA white paper, 16 years after ICH Q6A was adopted, takes us back to the original intent of ICH Q6A where specifications should focus on safety and efficacy, not batch data.
The relationship between patient centric specifications and batch data is illustrated in Fig. 1. The illustration (using product assay as an example) depicts the limits beyond which is deemed unsafe for patient (upper boundary or true maximum) or not efficacious for patient (lower boundary or true minimum). The batch data lie somewhere between these boundaries. The true minimum and maximum beyond which the drug product (DP) safety and/ or efficacy can be compromised are typically not known exactly—the opportunity to know those levels depends on deep knowledge of the product’s function in patients. Ideal patient centric specifications would be set inside the range of the true safety/efficacy boundaries. These limits are based on knowledge of the product’s true requirements (safety and efficacy) for patients. The use of prior knowledge can be useful in providing additional understanding of the criticality of product specific attributes and their impact on patient safety and efficacy. Together with clinical exposure and process experience, prior knowledge can be a useful tool in establishing appropriate clinically relevant specifications.
“Process Experience–Based” Approach and its Limitations
Current practices for setting specifications rely heavily on process experience from a very limited number of clinical batches, rather than in-depth knowledge of the product’s safety and efficacy in patients [14]. Typically the sponsor proposes specification limits based on the history of product attributes from very limited initial manufactured lots. The “process experience based” specifications can be derived in a number of ways, though the basic principle is similar by using some statistical analysis:
-
Statistical limits estimated to cover most of expected future results (e.g., tolerance interval limits)
-
Applying a mean ± k standard deviations, or
-
Using the range from the minimum and maximum limits attained during development.
It is understood and acknowledged that in cases where there is a high level of uncertainty, greater reliance on manufacturing process experience is merited. Per current Good Manufacturing Practice (CGMP) regulations and modernization in pharmaceutical industry, the manufacturing process consistency is monitored during the production of the drug substance and the drug product as part of the pharmaceutical Quality system. The “process experience based” approach does not answer the question whether the predicted variability will impact product safety/efficacy or not (i.e., it is just irrelevant). All these “process experience–based” approaches have the potential to present significant and deleterious consequences when applied to setting product specifications. These consequences include:
-
Risk that future batches will not meet criteria that were established based on a small number of development batches, for which the formulation and manufacturing process may not be optimized to achieve the required “in statistical control” state. This can result in unnecessary costly and timely reprocessing, reworking, rejection, and in some cases, recalls and shortages.
-
Manufacturers with poor control and more varied batch results will likely attain more flexible operating space with wider limits, whereas, manufacturers with tighter control and less batch variability during development stages may be forced to operate in a tighter space to ensure product meets the tighter specifications.
-
Manufacturing control strategies may be overly engineered with additional steps to ensure tight specifications can be met. This has the potential to add to the product cost through extra development costs, extended manufacturing times and increased capital resources.
-
Batch failures related to inappropriately tight specifications can minimize the power of Quality Metrics to identify meaningful and patient relevant system/process failures.
-
This could hinder the aspiration from regulatory and pharmaceutical industry to move towards “six sigma”. One of the fundamental reasons for the current two to three sigma quality performance seen in pharmaceutical manufacturing is because the acceptance criterion of the specification is set based on the observed variability rather than patient’s need (safety and efficacy) [15].
-
Regulators will continue to interpret batch data differently resulting in less harmonization of specifications. Country specific specifications lead to supply chain challenges where manufacturers must ensure each country receives the “right” batch of drug product in accordance with their specific specification. Supply chain flexibility is severely impacted. In addition, manufacturing to different specifications is generally not in line with good quality control practices.
-
Widening a specification post authorization for a global product is extremely costly and time-consuming when considering dossier preparation, country registration costs, and the long time period (1–5 years) to gain global regulatory approval (including ICH and non-ICH regions).
-
Opportunities to reduce manufacturing cost or otherwise make continual improvements are impeded by unnecessary regulatory barriers to change, especially globally divergent specification based on “process experience based” approach.
Continuing to develop the science and understanding of pharmaceutical products presents an opportunity for industry and regulators to increase their collaboration and deliver specifications that ensure patient needs continue to be met through the quality, safety, efficacy of new medicines and improves their availability with robust supply chains. This approach to patient centric specifications as a standard for pharmaceutical manufacturing supports the simultaneous attainment of two worthy goals: providing a reliable supply of safe and efficacious products to patients, while reducing manufacturing costs.
To limit the scope of this white paper, the authors will focus on how to establish patient centric specifications for drug substance and drug product impurities. Naturally, these fundamental principles can apply to other product critical quality attributes (CQAs) when the regulatory environment opens the door for this new paradigm. This paper will also discuss challenges the pharmaceutical industry is facing due to the regulatory interpretations of ICH Q6 divergence. To address these global challenges, the benefits of adopting patient centric specifications (PCS) early and throughout the product lifecycle will be discussed and how PCS can be established while maintaining the intended quality, safety, and efficacy of the product.
Establishing Patient Centric Specification for Drug Substance (DS)/Drug Product (DP) Impurity/Degradation Products
Patient centric specifications are a critical component to establishing control strategies and acceptance criteria for drug substance/drug product impurities. High levels of impurities/degradation products may result in the drug product being unsafe for patient, and may also reduce the drug efficacy as the active pharmaceutical ingredient amount is reduced. Then, why not try to develop material as pure as possible with no impurities? While certainly a laudable goal in isolation, it may have detrimental impacts in other aspects of manufacturing the drug substance. For example, the environmental impact would increase significantly as increased consumption of energy and raw materials and increased levels of waste would arise from the strive for absolute purity. Other product quality attributes, such as physical form, may be adversely impacted as one drives for more pure material. Finally, as yield goes down, and energy consumption and raw material usage increases, or the use of sensitive isolation techniques such as chromatography is required, the cost of production increases dramatically, possibly inhibiting the commercial viability of a highly efficacious drug which would have been a great benefit to patients.
Establishing the Safety of an Impurity
Establishing the safety of a drug substance impurity is central to establishing the acceptance criteria for a patient centric specification. FDA published a MAPP 5017.2 (Manual of Policies and Procedures) in 2018 which defines clinically relevant acceptance criteria as “a set of acceptance ranges to which an impurity should conform in order for the product to be safe and effective when used as labeled” [16]. This process typically begins with the identification and subsequent hazard classification of drug substance impurities. Table 1 describes the different types of drug substance impurities typically evaluated for safety. Actual impurities are those that are observed analytically and whose structure should be determined in the drug substance and/or drug product when their levels exceed the identification thresholds in Q3A/B [17]. Potential impurities (defined in Table 1), may have the potential to form and carry-through to the final drug substance. While there may be many “potential” impurities, the definitions in ICH M7(R1) help identify those potential impurities that would be critical to understanding the safety of the drug substance [18].
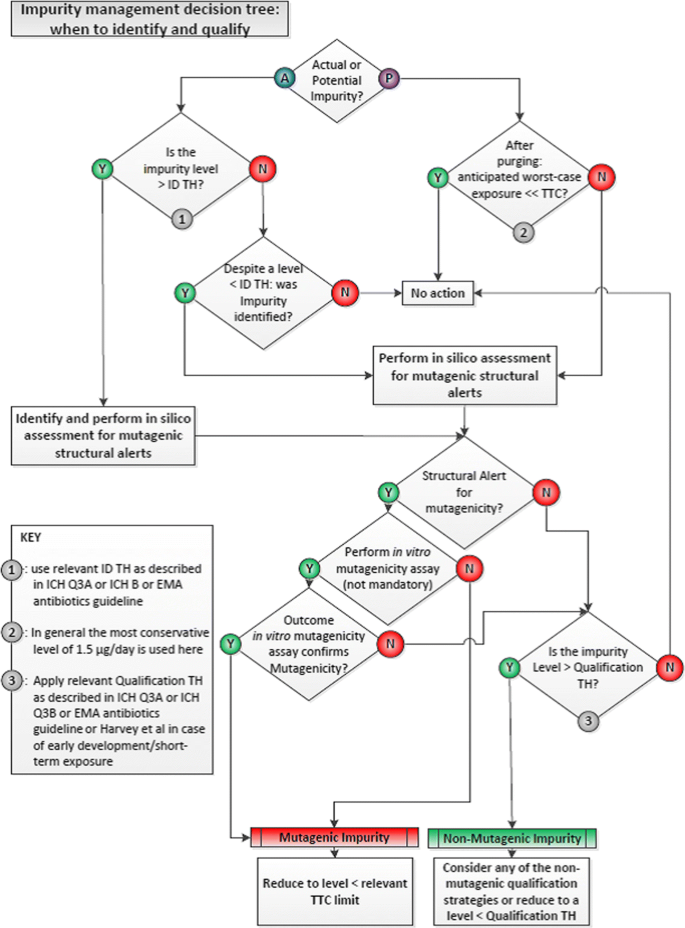
Figure 2 demonstrates the decision tree for identification, hazard classification, and qualification of drug substance impurities [19]. All actual impurities, if identified, are first assessed by literature and database searches. In the absence of prior knowledge, they are required to undergo a structure-based (i.e., “in silico”) assessment for mutagenicity. The structure-based assessment should use two different computational methods (i.e., rules and statistically-based) and an evaluation of the computational result by an expert. An expert can overrule any prediction but it must be justified and supported with appropriate data.
If an impurity is identified as being a structural alert for bacterial mutagenicity and exposure is not demonstrated to be below the threshold of toxicological concern (TTC), then a follow-up in vitro bacterial reverse mutation assay following OECD 471 methods (commonly referred to as the Ames assay) is used to confirm the prediction [20]. In some cases, an in vitro assay can be followed by an in vivo (whole-animal)–based mutation assay depending on the control of the impurity, nature of the alert, and response in the in vitro assay.
Once a compound is defined as “mutagenic” and/or carcinogenic, which is an ICH M7 Class 1, 2, or 3 compound, it should be controlled to its acceptable intake (AI) defined by either compound specific data or in the absence of such data, the TTC. Even for those impurities that are mutagens/potentially mutagenic, in parallel to any assessment of potential mutagenicity the actual potential for carryover at levels of concern may be assessed. ICH M7 offers a series of potential control options rather than simple reliance on end product testing (Option 1). Increasingly this is achieved through the use of worst-case purge calculations [21] without the need to generate analytical data, aligning to Option 4 within ICH M7.
The acceptable intake is highly dependent on the dataset of the compound. If a compound has only a mutagenic alert or is positive in a bacterial reverse mutation assay, then the appropriate TTC based on duration of exposure is used to derive the limit. The TTC is a conservative dose based on a large database of carcinogens which assumes the mutagenic compound is a highly potent carcinogen. If a compound is a mutagenic carcinogen, then those data should be used to derive a compound-specific acceptable intake, see Table 2. Where multiple mutagenic impurities are present, total mutagenic impurities should be limited. Methodology on how to calculate compound-specific acceptable intake and example common carcinogenic reagents are listed in Appendix 3 of ICH M7(R1) [18].
For any impurity defined as a Class 4 or 5, then the impurity is considered non-mutagenic and control can be based upon the principles defined in ICH Q3A/Q3B [17]. Qualification under ICH Q3A(R2) guidelines is the process of acquiring and evaluating data that establishes the biological safety of a drug substance impurity. The qualification threshold (QT), or percentage/dose below which qualification testing is not necessary, is dependent on the maximum daily dose of the active pharmaceutical ingredient as shown in Table 3. The QT is based on lifetime exposure and is applicable to commercial products.
The ICH Q3A(R2) guidelines provide general guidance on conducting qualification studies and setting limits based on those studies. In practice, drug sponsors implement these guidances based on internal procedures and regulatory feedback. The safety of an impurity is usually tested with the drug substance but can also be tested without the drug substance. Strategies can be used to increase the amount of impurities tested in animals such as spiking impurities or using relatively impure material. If testing with the drug substance, the qualified level of impurity is typically calculated from the no-observed-adverse-effect-level (NOAEL) of the drug substance and the relative contribution of the impurity as in Eq. 1.
where
- NOAEL:
-
No-observed-effect-level (mg/kg/day)
- BW:
-
Body weight (60 kg)
- MDD:
-
maximum daily dose
This process is conservative since the safe threshold of the impurity is likely calculated significantly below the actual safety threshold. Indeed, any toxicity in the study is most likely due to the pharmacology of the API tested; this is particularly true for anticancer treatments where establishing a NOAEL is often difficult. Since the safe threshold is unknown for the impurity, it is assumed to be at the low level tested in the toxicity study. Therefore, the qualified level is highly dependent on the concentration of impurity tested and the NOAEL tested in the study.
Some regulatory reviewers require adjustment of the NOAEL to a human equivalent dose (HED) in accordance with US FDA Guidance “Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult health volunteers” [22]. The concept is that scaling factors based on normalization of doses to body surface area can be used to extrapolate the metabolism differences between animals and man. The practical application is that the NOAEL is lowered to form the HED by a factor of 6.2 for rats, 12.3 for mice, 3.1 for monkeys, etc.; however, this has been applied inconsistently across regulatory reviewers since it is in direct contrast with ICH Q3A guidelines where they say “a level of qualified impurity higher than that present in a new drug substance can also be justified based on an analysis of the actual amount of impurity administered in previous relevant safety studies”. This language in ICH Q3A seems appropriate given the conservatism built around the impurity calculation and hence no further adjustment should be necessary in the context of an impurity. The safe impurity dose is calculated as a fraction of the API NOAEL, which is likely well below the true safe dose of the impurity. Converting to the HED for the safe starting dose is appropriate for the API but may be overly restrictive when applied to impurities.
Significant metabolites observed in animals and/or humans are generally considered qualified as impurities and do not require additional toxicity testing. Metabolites observed in humans that are greater than 10% of total drug-related exposure at steady state and at significantly greater levels in humans than the maximum exposure observed in toxicity studies require additional testing [23, 24]. Guidance on what testing needs to be performed is defined in USFDA 2016 guidance, but is beyond the scope of this discussion. It is important to highlight that these guidelines are intended to understand the safety risks of metabolites, thus the definitions in these guidance documents should not be intended for impurities. For example, a significant metabolite in relation to an impurity could be 5% since it is unlikely for a single impurity to exist at a level close to 5%. Also, the 5% metabolite in animals may well exceed a 5% exposure via the impurity because doses used for animals usually exceed the therapeutic dose, dependent on indication. Therefore, metabolite exposure at a NOAEL dose should be compared to impurity exposure to show the safety of the impurity via the metabolite to demonstrate that no additional toxicity testing is needed.
The ICH Q3A guidelines were intended to provide guidance for registration applications and not for clinical trial applications. Higher qualification thresholds have been historically applied by drug sponsors in early clinical trials, which was originally described in Zhang et al., 2012 as being 3× the ICH Q3A limits during early phase clinical trials [25]. Harvey et al., 2017 developed a scientific approach for deriving qualification thresholds in Phase I and II guidelines where the duration was < 6 months [26]. Based on three different toxicity datasets and application of a modified Haber’s rule, a qualification threshold of 5 mg/day, was derived. A quality-based concentration threshold of 0.7% was also defined for drug substance, similar to ICH Q3A, as small therapeutic doses could result in high qualification thresholds.
Another important guidance document in relation to impurity control is ICH S9. ICH S9 describes the nonclinical testing required for drugs indicated with patients that suffer from advanced cancer. Drugs that fall under ICH S9 are exempted from ICH M7 guidelines and “higher qualification limits” are allowed. For mutagenic impurities present in an anti-cancer treatment intended for an S9 defined population, the threshold of toxicological concern (TTC) approach for calculating limits is not recommended; however, ICH S9 does not provide specific guidance on higher limits for mutagenic impurities nor does it comment on any modification to ICH Q3A, particularly qualification thresholds. In terms of qualification studies, the pharmacology associated with the active agent in anticancer medicines is such that it is often difficult to establish a NOAEL level for an impurity. One practical approach is apply the qualification threshold as described by Harvey et al., 2016 for short-term administered compounds (< 6 months) of 5 mg/day or 0.7%, whichever is lower. This is based on the same principle as Haber’s Law applied to mutagenic impurities, differing though in the application of a conservative derivation of the Law based on the potential acute toxicity of an impurity.
Generating Limits for Impurities with Established Toxicity Data
There are several compound classes with established datasets such as solvents, metals, and common reagents/impurities. In these cases, the toxicity data must be used to derive the limit. This section will describe how limits are toxicologically developed for these impurities.
ICH Q3C(R6) describes principles and methods for deriving permitted daily exposures (PDEs) for solvents and ICH Q3D describes the same process for elemental impurities [27, 28]. Both approaches are similar, but differences exist due to the nature of the chemical. The PDE is derived as follows:
where
- NO(A) EL:
-
No-observed-effect-level or No-observed-adverse-effect-level
- F1:
-
A factor for extrapolation between species
- F2:
-
A factor of 10 to account for variability between individuals
- F3:
-
A variable factor to account for toxicity studies of short-term exposure
- F4:
-
A factor that may be applied in cases of severe toxicity, e.g., non-genotoxic carcinogenicity, neurotoxicity, or teratogenicity
- F5:
-
A variable factor that may be applied if the NOEL is not established
- BW:
-
An arbitrary body weight of 50 kg (assumes a smaller person)
The PDE for solvents (ICH Q3C) was intended for all routes of administration given the high inhalation and/or oral bioavailability of solvents. The PDE for metals is route specific, with PDEs developed for oral, parenteral, and inhalation routes of administration based on low bioavailability of metals by a particular route and/or the specific toxicity observed from a specific route of administration.
ICH Q3C has three classes of solvents, dependent on their toxicity and shown in Table 4. Also ICH Q3D has three classes but Class 2 is subdivided into 2A and 2B based on the probability of occurrence in human pharmaceuticals (Table 5).
ICH M7(R2) in its appendix provides guidance on developing limits for mutagenic compounds and carcinogens [18]. For mutagenic carcinogens (i.e., Class 1 compounds) where a threshold for mutagenicity has not been established, an allowable intake (AI) is developed to protect for a 1 in 100,000 excess risk of cancer. The AI is developed from the cancer endpoint such as the TD50 (50% tumor incidence over background) for the most sensitive tumor type, animal species, and sex where preference is made for the more robust carcinogenicity studies. The AI is calculated as follows:
Bercu et al., 2018 is also another resource which has developed PDEs and AIs for common reagents and impurities in pharmaceuticals using methodology described in ICH Q3C(R6), ICH Q3D, and ICH M7 (R2) [29]. The publication also contains Supplementary Materials which describe the toxicity data and scientific process by which all toxicology limits were established. Each monograph was authored by a toxicologist and peer-reviewed to ensure scientific rigor for each toxicology limit.
The ICH guidelines provide an established scientific framework for the identification and qualification of impurities and are the foundations for patient centric specifications. By taking the existing ICH guidance, combining with enhanced product and process understanding, will allow specifications to be defined based on the science and knowledge. This presents the opportunity to engage with regulators to agree the limits that will ensure patient needs continue to be met in the future. Unfortunately, recent negotiations between health authority and applicants (company) related to proposed marketing applications show that on a global level, batch experience, even when limited, plays an overwhelming role in developing impurity specifications rather than in-depth knowledge of the product’s safety and efficacy in patients.
Industry Case Studies
To illustrate the challenges that pharmaceutical industries are facing for the global regulatory requirements, three case studies are presented in this section. The case studies do not identify the specific reviewing country, rather they are referred to generically as Regions 1, 2, 3, etc. For all three cases, the actual country is consistently identified with the same region number. For example, Country A would be referred to as Region 1 in all three case studies. This is important as it shows that not only do we see between country differences, but we also see within country differences, when it comes to impurity review.
Case Study-1
The first case study represents a global registration of a small molecule over the 2014–2016 timeframe. For this example, the registration experience for one inorganic impurity attribute, palladium, in the drug substance, is shared below (Table 6).
Palladium is commonly used as a catalyst in small molecule synthesis and has historically been controlled at very low levels in pharmaceuticals. Prior to the publication of ICH Q3D, there was limited, globally accepted guidance available to justify safe limits for palladium. As a result, manufacturers were expected to control the impurity to the lowest levels possible. This control required extensive investment in the development and implementation of robust manufacturing processes to achieve these low levels that investment continues today for many old and new commercialized products. ICH Q3D, now providing a basis to establish safety-based specifications, can have a positive impact on where and how manufacturers invest in process development to ensure a safe product [28]. The full benefits of ICH Q3D can only be realized if the guideline is adopted globally.
In the above case study, the control of palladium was the subject of concern by one ICH country. Despite the safety based justifications offered to support a specification of 500 ppm vs the ICH Q3D PDE of 800 ppm, this country requested a tighter specification [28]. The regulator concluded that palladium offered no therapeutic benefit to the patient and therefore, requested the manufacturer to tighten the acceptance criterion for palladium based on batch analysis data and manufacturing capability in the event that higher doses will be used for other indications or as combination use with other drug products. At the time of the registration, there was no discussion or plan that higher doses would be marketed so the dose concern from the country appeared unfounded. The second concern related to co-dosing and the potential for an additive exposure to palladium; however, it is impossible for the applicant to consider the levels of palladium in other drug products that are not within their own portfolio, never mind develop a robust control strategy to control to an unknown limit.
As a result, the manufacturer agreed to tighten the specification to 200 ppm. Given the historical batch data showed some lots were close to 200 ppm, the manufacturer was concerned that there was now a real risk of batch failures using the current synthetic process. To ensure future batches would meet the tighter limit, a palladium scavenger step was developed, validated, and introduced post-approval. The additional measure of control cost the research and development organization many months of development time and added to the overall cost of the commercial process and time to produce each batch of API.
Case Study-2
Case Study 2 is for the same molecule as Case Study 1, a global registration of a small molecule over the 2014–2016 timeframe. In this case, the drug product specification for an impurity, that was also a significant metabolite, was the subject of concern in one ICH country. The manufacturer attempted to justify the proposed specification using a safety-based argument showing that the impurity, a metabolite, would be at a greater level in-vivo than the proposed specification. Over numerous rounds of back and forth communication with the health authority, the reviewer insisted the specification be based on batch data and the mean + 3 standard deviations, rather than accepting the clinical relevance argument. The final specification fell in between the originally proposed specification and the batch data results (Table 7).
Case studies 1 and 2 highlight the quality review differences in the ICH regions. In Case Study 1, only Region 1 raised a concern with a safety based specification, yet in Case Study 2, that same region accepted the safety based argument for the metabolite. Similarly, Region 3 accepted the safety based, ICH Q3D rationale, for palladium in the first Case Study, but rejected a safety-based argument for the metabolite in Case Study 2. It is interesting to also note that ICH Region 3 requested to see the specifications from the other regions as part of their review, so they were aware of the differences.
The small molecule case studies discussed above clearly point to not only differences between regions but even differences within a region where the manufacturer provided clear safety justifying the proposed specification limit.
Case Study-3
Case study 3 is for antibody drug conjugate (ADC) comprised of a monoclonal antibody (IgG4) conjugated via lysine chemistry to a calicheamicin derivative. It is currently approved in several markets for an oncology indication.
A recent white paper by Gong, et al. highlights considerations with respect to establishing acceptance criteria for small molecule impurities in ADCs [31]. As regards free payload, the basis of defining an appropriate acceptance criterion is principally based on assurance of safety.
A ≤ 4.0% limit for the unconjugated payload in drug product is supported by non-clinical safety data. Unconjugated payload at 4.0% equates to a safety margin greater than an order of magnitude (1/25) from the NOAEL for unconjugated payload. Further, non-clinical data provides evidence that the toxicity profile of the molecule principally relates to non-specific binding of the conjugate. From this perspective, the limit proposed was viewed as well justified and was the proposed basis of defining the acceptance criteria in the initial applications to several markets.
In addition to this limit, relevant batch data comprising eight lots was subjected to a statistical analysis and shows that based on a tolerance interval of the batch data, the levels should remain at ≤ 3.1%. The actual range of observed data of relevant batches showed a maximum of 2% unconjugated calicheamicin from a total of eight clinical drug product lots.
During the negotiations in the review and approval process with different health authorities, the final established acceptance criterion for unconjugated calicheamicin differed as did the basis of what was considered appropriate to define the acceptance criterion (Table 8).
This outcome and the experience during the review of Case Study-3 illustrate several interesting points:
-
1.
There is clearly divergence within the different health authorities around the appropriate basis of establishing the acceptance criterion for this particular attribute. This seemingly stems from different views regarding the degree of uncertainty in the data, the degree of risk of the attribute and potential opportunities to minimize safety risks.
-
2.
There was a strong interest in the majority of the health authorities to base the acceptance criterion on the batch data itself, rather than consider a scientific justification grounded in the safety data.
-
3.
The majority of markets were in agreement in using a statistical range around the batch data to define the acceptance criterion.
-
a.
One health authority accepted the safety justification.
-
b.
One health authority viewed the safety consideration for the unconjugated payload as of such concern that the specification should be further tightened to the maximum of what was exposed in the clinic trials. This was in spite of the large majority of the payload being attributed to the molecule itself.
-
c.
In contrast, another health authority required additional doses to be evaluated in a post marketing study.
-
a.
-
4.
Specification negotiations for commercial products go on in parallel with the initial application reviews which affords limited opportunity and significant pressure to come to a consensus to get the product approved.
-
5.
It is not clear that the differences in quality standards actually bring any value to patients or lead towards a more or less safe exposure with the product. We do know however that varied quality standards for a product increases complexity in pharmaceutical quality systems, supply and distribution plans. This complexity in turn leads to increased costs and potential for issues with supply continuity.
It is worth noting that FDA MAPP 5017.2 “Establishing Impurity Acceptance Criteria As Part of Specifications for NDAs, ANDAs, and BLAs Based on Clinical Relevance” was issued since US approval of the product [16]. In the MAPP, it states that for impurities where there is a high level of uncertainty or where an impurity could be a surrogate for other impurities (free toxin for a toxin-conjugated drug product was used as an example) the impurity acceptance criteria should include greater consideration for manufacturing process capability. While it is understood and acknowledged that in cases where there is a high level of uncertainty, greater reliance on manufacturing process capability is merited, it is unclear why the totality of non-clinical and clinical data was not viewed as sufficient to support acceptance criteria based on NOAEL given other impurities were effectively covered such that the unconjugated payload was not a surrogate for other impurities in the control strategy. It would be helpful to state that scientific data relating to the impact of the given attribute is most meaningful and efforts to reduce uncertainty, even for complex products, merit consideration as the basis of defining appropriate acceptance criteria.
These recent case studies highlight the divergence of regulatory acceptance to patient centric specifications from region to region and even within one region. Industry, regulators, and arguably, the patient, stand to benefit from a harmonized approach to establishing impurity specifications based more on safety and efficacy of the product, rather than the batch variability data.
Regulatory Strategies During the Product Lifecycle
Several approaches may be considered for specification setting based on patient needs in the drug development, registration, and post-approval phases of a product life-cycle. It is critical that regulators and industry strive to a common strategy to ensure patients have uninterrupted access to high quality, safe, and affordable drugs. The overarching goal is to maintain patient-centricity throughout the product lifecycle.
During the early phases of drug development, Phases 1 and 2, demonstrating the safety of the compound under study is the primary objective. Correspondingly, the safety of impurities should underpin the control strategy during the early phase of development. During these phases, API and drug product-specific batch and stability data will be extremely limited. The fundamental chemistry in drug substance synthesis is often not locked in until registration stability batches, as a result of which, Phase 1 and 2 batches may not be representative of Phase 3 batches. Further process optimization in Phase 3 following the manufacturing of registration stability is possible, while retaining the same fundamental chemistry. An understanding of variability of the impurity profile through scale-up and validation is a frequent challenge.
In general, product development is inherently a very dynamic process where drug substance and drug product processes are in constant change. Rarely, if ever, is one process developed in Phase 1 that ends up as a registration process. Depending on the length of development and disease state studied, very few batches may be produced as part of Phase 3. Using batch experience to establish specification criteria at this stage can/will result in batch rejections or additional regulatory amendments to widen acceptance criteria to qualified safe limits. Additionally, adoption of narrow specifications based on limited experience can negatively impact clinical study initiation or study continuation where regulatory approval must be gained to use batches in the clinic that have higher impurity levels than previously seen. Leveraging prior knowledge from analogous molecules, processes, platform technologies, etc. allows for the creation of a broader data set contributing to a more robust understanding of criticality of material attributes and process parameters and their influence on impurity levels.
As previously discussed, Harvey et al. describes a simplified approach for supporting the safety of non-mutagenic impurities in new drug substances and drug products used in the early stages of clinical development where ICH Q3 guidelines are not applicable and study duration is limited [26]. With this approach, the API control limits of < 1 mg/day lifetime dose, or 5 mg/day or 0.7%, whichever is lower, for clinical studies of less than 6 months duration, would not represent a safety concern. Equally, for drug product limits of 5 mg/day or 2% (whichever is lower) would be also not present safety concerns in early clinical studies. This simplified approach to establishing non-mutagenic impurity specifications for organic impurities in early clinical studies, which is summarized in Table 9, may offer an alternate approach where specific toxicological studies are not available. As a product moves into larger and longer clinical trials, such as phase 3, specifications should begin to be developed based on non-clinical toxicological studies. ICH S9 should be considered and applied from the start of development for disease indications where wider control strategies may be appropriate [32]. At registration and post-approval, ICH Q3A/B will continue to apply. Importantly, in post-approval, where more product experience is gained, there are situations where new impurities or higher levels of existing impurities may need to be considered. Again, non-clinical toxicological studies in accordance with ICH Q3A/B, and not historical batch data, should support changes to the impurity specifications.
At the time of product registration, the applicant is expected to establish specifications aligned with ICH Q3A/B guidelines and/or local country regulations. Batch manufacturing experience is generally very limited at the commercial manufacturing facility when filing a marketing application. From the case studies, it is clear that the industry has experienced a wide range of opinions by the individual ICH countries on what is an appropriate impurity specification. Some ICH countries appear to be more accepting of impurity limits based on toxicological data, whereas, other ICH countries adhere to ICH Q6 A/B Decision Tree and require specifications be established based on historical batch data ± 3 standard deviations. Relying on batch data at this early point in the product lifecycle, where little if any process and material variability has been introduced, leads to an artificially tight specification where an appreciable risk of batch rejection is undertaken. While the specification is tighter, the quality of the product to an individual patient may not be meaningfully better. The different specification tolerances from the ICH countries have required companies to establish multiple specifications for the same product coming from the same manufacturing line and same manufacturing plant. This adds to the complexity of the supply chain and potentially puts the supply to some countries at risk if future batches cannot meet the tighter specification.
Following the approval of the product, additional commercial manufacturing experience can introduce variability that was not studied during development and which could have an impact upon impurity levels. Process improvements, site transfers, and analytical methodology may trigger the need to re-evaluate an impurity limit. Often, such changes result in no change in the impurity profile, but on occasion there is a new or higher level of an impurity that requires attention. In these cases, where reasonable efforts cannot ensure the product will meet the current impurity specification, a post approval variation (in general it is considered as significant change-prior approval supplement (PAS) or Type II filing) must be prepared and defended across all the markets. Again, regulators often align the new specification with the batch data with minimal consideration for the safety qualification level. The evolution of the concept of patient centric specifications described throughout, aided by the potential framework defined within ICH Q12 Step 2 could dramatically reduce this burden while still maintaining quality, safety, and efficacy of the product [33].
Regardless of the phase of development, it is imperative to consider and balance impurity levels with target potency and assay specifications. At registration, contemporary small molecule products are generally expected to meet an API potency range of 98.0–102.0% and a drug product assay range of 90.0–110.0% (at a minimum—95.0–105.0 in some regions) through expiry. Wider ranges can be appropriate in the early stages of development. Ultimately the total impurity acceptance criterion should not compromise the potency and assay limits, to ensure the patient receives the appropriate amount of active to be efficacious.
Summary and Future Perspectives
In this paper, we remind readers of several ICH guideline documents such as ICH Q3A, Q3B, Q3C, Q3D, Q6A, Q6B, M7, and ICH S9 which are related to the drug substance and drug product impurity limit setting. In particular, ICH Q6A clearly states that “specifications should focus on those characteristics found to be useful in ensuring the safety and efficacy of the drug substance and drug product”; however, recent negotiations between health authority and applicants (company) related to proposed marketing applications show that on a global level, batch experience, even when limited, plays an overwhelming role in developing impurity acceptance criteria rather than clinical relevance. The drawback of such practice and the great need to establish patient centric specifications (PCS) are highlighted. Secondly, this paper reviews approaches on how to establish patient centric criteria for drug substance and drug product impurity limits based on the principles outlined in ICH guideline documents and scientific literature. Three case studies are presented to illustrate the challenges in establishing PCS and the divergence of regulatory acceptance to such specifications. Lastly, we propose the approaches that can be considered for specification setting based on clinical relevance in the drug development, registration, and post-approval phases of a product life-cycle.
As presented in the main body sections of this paper, the over reliance or emphasis on batch data, which is but one component in the justification of a specification, or process capability based upon limited data, is not fully aligned with the principles outlined in the current ICH guideline documents, and often does not consider all of the information provided by the filing applicant in justification of the specification. The patient centric specification based on safety and efficacy, rather than the variability observed in limited development and clinical batches, would be a solid foundation to ensure a safe, efficacious, and high-quality drug product will be available, without interruption of the supply.
The scope of FDA MAPP 5017.2, titled Establishing Impurity Acceptance Criteria as Part of Specifications for NDAs, ANDAs, and BLAs Based on Clinical Relevance [16] is to provide reviewers guidance on the principles and approaches for establishing drug substance and drug product impurity acceptance criteria for non-mutagenic impurities in NDAs, ANDAs, and BLAs, based on the consideration of clinical relevance. The procedure states that the principles that guide the development of a specification can be impacted by the assessment of risk to safety and efficacy based on context of use as well as other factors, such as clinical experience. Furthermore, the procedure emphasizes that the types of data and information should take into consideration the clinical impact of impurity levels, as opposed to manufacturing process capability, to ensure the acceptance criteria are clinically relevant. Although the scope of the MAPP is limited to impurities, this policy and procedure demonstrates substantial progress in thinking in terms of justifying and setting specifications that are clinically relevant or patient centric. The document should facilitate prioritizing dialog between filing applicants and the regulatory authorities in regard to specifications based upon safety, efficacy, and ultimately clinical relevance as it is recognized by all that impurity acceptance criterion requires careful consideration and cannot be established using one definitive approach.
The rapid uptake of new science in the industry resulting in the advent of new modalities such as, but not limited to, oligonucleotides, ADCs, CAR-T therapy, and personalized medicines will continue to strain the current justification of specification process between health authorities and applicants. This change will usher in the need for a new way of thinking and will rely extensively upon patient centric specifications. It is probably fair to state that both regulators and industry alike could envision improvements in justifying and setting of specification acceptance criteria. The authors believe that there are opportunities to improve understanding and reduce uncertainty regarding the justification and setting of specifications as exemplified by the case studies presented in this paper. The authors offer the following recommendations to improve transparency in the process:
-
Industry to leverage public venues for dialog with health authorities such as FDA, EMA, and PDMA through conferences, roundtable discussions, and publication of papers. One output of this endeavor could be to gain alignment of industry and health authorities on terminology and strategies related to patient centric specifications with the output being joint health authority industry publications.
-
Industry to advocate for the need for ICH to issue new guidance(s), points to consider, or Questions and Answers related to setting patient centric specifications. Opportunities exist for Q&A or Points to Consider for application of existing guidance for new modalities that would benefit industry by reducing uncertainty in pharmaceutical development programs.
-
Industry to participate in conferences in both ICH and non-ICH regions to inform through dialog about the benefits of patient centric specifications and to provide examples of benefit.
-
Industry to nurture the concept of patient centric specifications, by using ICH terminology, use of common terms and language, and justifying and setting specifications based upon impurity qualification, knowledge of product, and needs of patient (safety and efficacy). To engage with regulators, at earliest opportunity, to share the strategy and additional data as required to demonstrate the rigor of the specification.
In conclusion, regulatory authorities and industry both need to foster discussions and forums that focus on getting medicinal products that are safe and effective to the patient sooner to meet unmet medical needs while minimizing supply interruption concerns.
Abbreviations
- PCS:
-
Patient centric specifications
- CRS:
-
Clinically relevant specifications
- ICH:
-
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use
- US FDA:
-
United States Food and Drug Administration
- EMA:
-
European Medicines Agency
- PMDA:
-
Pharmaceuticals and Medical Devices Agency
- ISPE:
-
International Society for Pharmaceutical Engineering
- ADC:
-
Antibody drug conjugate
- MAPP:
-
Manual of Policies and Procedures
- CAR-T:
-
Chimeric antigen receptor (CAR) T cell therapy
- AI:
-
Acceptable intake
- TTC:
-
Threshold of toxicological concerny
- QT:
-
Qualification threshold, NOAEL: no-observed-adverse-effect-level
- HED:
-
Human equivalent dose; PDE: permitted daily exposure
References
US Food and Drug Administration (USFDA). 21st Century Cures Act. Page last updated 3/29/2018. https://www.fdagov/regulatoryinformation/lawsenforcedbyfda/significantamendmentstothefdcact/21stcenturycuresact/defaulthtm. 2018.
US Food and Drug Administration (USFDA). Submission to congress: Food and Drug Administration work plan and proposed funding allocations of FDA innovation account. Required by section 1002 of the 21st Century Cures Act (Public Law 114–225). 2017.
International Conference on Harmonization (ICH). Q6A: specifications: test procedures and acceptance criteria for new drug substances and new drug products: chemical substances. 1999.
International Conference on Harmonization (ICH). Q6B: specifications: test procedures and acceptance criteria for biotechnological/biological products. 1999.
Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–91. https://doi.org/10.1007/s11095-007-9511-1.
US Food and Drug Administration (USFDA). Guidance for industry: PAT - a framework for innovative pharmaceutical development, manufacturing, and quality assurance. 2004.
Baum RG. ICH Q8/9/10 - a new paradigm: why Q8/9/10 - how are they different from Q1-Q7? Presentation at workshop on implementation of ICH Q8/Q9/Q10 and other quality guidelines, Beijing. 2008.
International Conference on Harmonization (ICH). Q9: quality risk management. 2005.
International Conference on Harmonization (ICH). Q10: Pharmaceutical quality system. 2008.
International Conference on Harmonization (ICH). Q8(R2): pharmaceutical development. 2009.
Sharp SS. Establishing clinically relevant drug product specificaitons: FDA perspectives. Presentation at AAPS Annual Meeting. 2012.
Lostritto R Clinically relevant specificaitons (CRS): a regulatory perspective. Presentation at Conference on Evolving Product Quality. 2014.
US Food and Drug Administration (USFDA). White paper: FDA pharmaceutical quality oversight. 2015.
Dong X, Tsong Y, Shen M. Statistical considerations in setting product specifications. J Biopharm Stat. 2015;25(2):280–94. https://doi.org/10.1080/10543406.2014.972511.
Yu LX, Kopcha M. The future of pharmaceutical quality and the path to get there. Int J Pharm. 2017;528(1–2):354–9. https://doi.org/10.1016/j.ijpharm.2017.06.039.
US Food and Drug Administration (USFDA). MAPP 50172: Policies and procedures. Office of pharmaceutical quality. Establishing impurity acceptance criteria as part of specifications for NDAs, ANDAs, and BLAs based on clinical relevance. 2018.
International Conference on Harmonization (ICH). Q3A(R2): impurities in new drug substances. 2006.
International Conference on Harmonization (ICH). M7(R1): assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. 2017.
Teasdale A, Elder D, Harvey J, Spanhaak S. Impurities in new drug substances and new drug products. In: Teasdale A, Elder D, Nims RW, editors. ICH quality guidelines. NJ: John Wiley & Sons Inc.; 2018. p. 167–98.
Organization for Economic Co-operation and Development (OECD). Test no. 471: bacterial reverse mutation test. 1997.
Teasdale A, Elder D, Chang SJ, Wang S, Thompson R, Benz N, et al. Risk assessment of genotoxic impurities in new chemical entities: strategies to demonstrate control. Org Process Res Dev. 2013;17(2):221–30. https://doi.org/10.1021/op300268u.
US Food and Drug Administration (USFDA). Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005.
International Conference on Harmonization. M3(R2): guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. 2009.
US Food and Drug Administration (USFDA). Guidance for industry: safety testing of drug metabolites. 2016.
Zhang S, Coutant M, O'Connor D, Szulc M, Trone MD, Swanek F, et al. Early development GMPs for small-molecule specifications: an industry perspective (part V). Pharm Technol. 2012;36(10):1–5.
Harvey J, Fleetwood A, Ogilvie R, Teasdale A, Wilcox P, Spanhaak S. Management of organic impurities in small molecule medicinal products: deriving safe limits for use in early development. Regul Toxicol Pharmacol. 2017;84:116–23. https://doi.org/10.1016/j.yrtph.2016.12.011.
International Conference on Harmonization (ICH). ICH Q3C(R6): impurities: guideline for residual solvents. 2016.
International Conference on Harmonization (ICH). Q3D: guideline for elemental impurities. 2014.
Bercu JP, Galloway SM, Parris P, Teasdale A, Masuda-Herrera M, Dobo K, et al. Potential impurities in drug substances: compound-specific toxicology limits for 20 synthetic reagents and by-products, and a class-specific toxicology limit for alkyl bromides. Regul Toxicol Pharmacol. 2018;94:172–82. https://doi.org/10.1016/j.yrtph.2018.02.001.
European Medicines Agency (EMA). Guideline on the specification limits for residues of metal catalysts or metal reagents. EMEA/CHMP/SWP/4446/2000. 2008.
Gong HH, Ihle N, Jones MT, Kelly K, Kott L, Raglione T, et al. Control strategy for small molecule impurities in antibody-drug conjugates. AAPS PharmSciTech. 2018;19(3):971–7. https://doi.org/10.1208/s12249-017-0943-6.
International Conference on Harmonization (ICH). S9: nonclinical evaluation of anticancer pharmaceuticals. 2009.
International Conference on Harmonization. Q12 draft guideline: technical and regulatory considerations for pharmaceutical product lifecycle management. 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bercu, J., Berlam, S.C., Berridge, J. et al. Establishing Patient Centric Specifications for Drug Substance and Drug Product Impurities. J Pharm Innov 14, 76–89 (2019). https://doi.org/10.1007/s12247-018-9366-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-018-9366-5