Abstract
We summarize the results of studies on quasicrystals (QCs) at extreme conditions over the last 4 decades with particular emphasis for compositions falling in the Al-based ternary system as the closest to those of quasicrystals discovered in nature, such as icosahedrite and decagonite. We show that, in contrast with what thought in the past, both pressure and temperature act to stabilize QCs, for which a clear phase transition to either crystalline approximants or amorphous material has been limited to very few compositions only. Such stabilization is proved by the compressibility behavior of QCs that resembles that of the pure constituent metals. Additional remarks come from the experimental observation of QC formation at high pressure and temperature in both static and dynamic experiments. These results seem, in conclusion, to suggest that the occurrence of QCs in nature might be more a rule rather than an exception.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Quasicrystals (QCs), generally defined as quasiperiodic solids characterized by unconventional crystallographic rules (Levine and Steinhardt 1984), find applications in industry due to their physical properties, light absorption, reduced adhesion and friction, thermal insulation, coating on mechanical devices, etc. (Dubois 2012, 2023). The investigation of the physical and chemical properties of QCs appears also relevant in the field of planetary geology despite their rare occurrence in nature. Indeed, the Khatyrka meteorite, a CV3 carbonaceous chondrite discovered in the Koryak mountains, Eastern Russia (Bindi et al. 2012; MacPherson et al. 2013), represents, to date, the only natural sample that has led to the discovery of icosahedral (i)-Al63Cu24Fe13, then named icosahedrite, the first naturally occurring QC (Bindi et al. 2009, 2011). The discovery was followed by the finding of the second natural QC, Al71Ni24Fe5, named decagonite for the internal decagonal symmetry (Bindi et al. 2015a, b), and, very recently, a third, still unnamed i-QC, Al62Cu31Fe7 (Bindi et al. 2016). The latter QC is quite exceptional owing to its Fe-poor composition outside the stability field of QCs in the Al–Cu–Fe ternary system (Bancel 1999) and represents the first example of a composition discovered in nature prior to being discovered in the laboratory. The meteorite containing natural quasicrystals shows evidence indicating high pressures (P) and temperatures (T) (Hollister et al. 2014; Lin et al. 2017).
Besides the finding of icosahedrite as intergrowth within silicates that can be taken as proof of their chemical equilibrium, icosahedrite was reported to coexist with two high-pressure phases, i.e., stishovite and ahrensite (Bindi et al. 2012; Hollister et al. 2014). The former being stable between 8 and 60 GPa (Swamy et al. 1994; Kuwayama 2008), while the latter, representing the high-pressure (HP) polymorph of Fe2SiO4 (Ma et al. 2016), is stable above ~ 5 GPa (Yagi et al. 1987) up to ~ 23 GPa (Presnall 1995).
Along with the coexistence of HP minerals, the presence of dendritic quenched textures formed by metallic liquids (Hollister et al. 2014; Lin et al. 2017) can be interpreted as evidence of the melting-driven formation of QCs that likely also led to the formation of additional Al–Cu–Fe crystalline phases such as khatyrkite, cupalite, hollisterite, kryachkoite, and stolperite (Ma et al. 2017).
To corroborate these observations, laboratory experiments were carried out. They included: (1) testing the stability of QCs at high P and T; (2) phase equilibria studies within chemical systems representative of icosahedrite, for instance, within the Al–Cu–Fe system; and (3) constraining the P–T conditions for melting of QC.
In the frame of an international conference on quasicrystals held at the Accademia dei Lincei in November 2022 (Bindi and Parisi 2023), we took the occasion to review past and recent experimental studies on the HP-T stability of synthetic QCs, with particular attention to the compositions of interest for planetary geology.
2 Al-based quasicrystals at high pressures
The general interest for QCs relies on their diverse physical properties that result in potential wide industrial applications such as hydrogen storage, hydride battery materials, and coating of soft metals (Dubois 2000, 2012, 2023). Beyond the highly debated topic whether QCs are stabilized by entropy or energy, their investigation under HP allows to monitor changes of the atomic arrangement resulting into phase transitions as well as changes in their physical properties that might be of inspiration for further industrial applications. Al-based QCs are of particular interest in this review as their composition resembles that found in nature (Bindi et al. 2009, 2011, 2015a, b, 2016). Table 1 is a summary of the QC compositions that have been investigated at high P so far, along with their elastic parameters (K0 in GPa and KI0) resulting from fitting the volume data collected up to the maximum reported P with either a Murnaghan or a Birch–Murnaghan Equation of State (EoS). Table 1 represents, therefore, an updated version of what was given by Krauss and Steurer (2004). It reports the stoichiometry for each QC and the latest experimental data referred exclusively to binary and ternary Al-based QCs due to their similarity with both natural icosahedrite and decagonite. As it can be seen from Table 1, the bulk modulus, K0, varies between 79 GPa for i-Al62Cu25.5V12.5 (Ponkratz et al. 2001) and 180 GPa for i-Al70.5Pd21Re8.5 (Sadoc et al. 1998), the discrepancy being likely related to the different chemical composition.
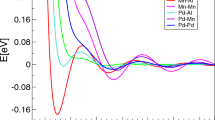
A couple of years after the experimental finding of icosahedral Al86Mn14 by Shechtman et al. (1984) and the theoretical prediction by Levine and Steinhardt (1984), the first HP investigation of i-QC up to 14 GPa by monitoring changes in resistivity of i-Al78Mn22 and i-Al86Mn14 was performed by Parthasarathy et al. (1986). The authors reported a QC-to-crystalline transition (plus the occurrence of pure Al) at 5 and 9.2 GPa, respectively. Later, a powder XRD study was performed on the binary i-Al78Fe22 up to 8.3 GPa by Jaya et al. (1987) using a metal anvil clamp cell and NaCl as pressure medium. They proved the stability of the i-QC with an anisotropic behavior under compression without providing elastic parameters. More HP data became later available for binary i-AlMn alloys by both conventional powder XRD (Sato-Sorensen and Sorensen 1989) and synchrotron energy-dispersive XRD (Johnson et al. 1988), which allowed a much better resolution other than to shed light on the effects of composition on the bulk modulus. Figure 1a shows the volume change, V/V0, with the applied P (GPa) for each of the three known icosahedral QC (i-Al78Mn22, i-Al74Mn26, and i-Al86Mn14) for which a K0 varying between ~ 118 and 148 GPa can be seen. The diagram also shows the compressibility of the two pure metals (Al EoS from Dewaele et al. 2004; Mn EoS from Fujihisa and Takemura 1995) and for the crystalline AlMn alloy (Sato-Sorensen and Sorensen 1989) for comparison. The V/V0 of the i-QCs appears related to the compressibility of the pure metals. Noteworthy, i-QCs result less compressible than the crystalline α-AlMn (K0 of ~ 85 GPa; Sato-Sorensen and Sorensen 1989).
Pressure–volume data for a QCs known within the binary Al–Mn system; b QCs with icosahedral and decagonal symmetry within the AlYCu system with Y = Ru, Co, and Li; c QCs within the AlCuZ with Z = Ru, Cr, Mn, and V; d QCs with icosahedral symmetry and an approximant within the system Al–Cu–Fe system. The curves obtained from these studies are drawn within the investigated P range considering that no structural phase transformation has been shown. For each diagram, the P–V data of the pure metals are reported for comparison with references given in the text
Further Al-based QCs are those in the ternary Al–Y–Cu system with Y = Li, Ru, and Co (Akahama et al. 1989, 1991; Krauss and Steurer 2004). Among them, Al60Li30Cu10 possesses a decagonal symmetry, for which the HP behavior was investigated using diamond anvil cell (methanol + ethanol ± water as pressure medium) both with a conventional powder diffractometer and angle-dispersive synchrotron radiation up to about 30 GPa. Once again, the bulk moduli of the i-Al60Li30Cu10, i-Al68Ru17Cu15, and d-Al60Li30Cu10 are observed to vary from 70 to 150 GPa and the V/V0 is shown in Fig. 1b along with the compressibility of pure Co (Fujihisa and Takemura 1996), Cu (Dewaele et al. 2004), Ru (Anzellini et al. 2019), and Li (Hanfland et al. 1999). The dependence of the QCs compressibility from that of the pure constituents appears obvious and can be used to predict preliminarily the physical behavior of a specific QC alloy. Interestingly, Akahama et al. (1989) reported amorphization followed by crystallization in the case of i-Al60Li30Cu10. This observation followed the previous report by Parthasarathy et al. (1986) of QC-to-crystal transition suggesting possible metastability of the QC out from their P–T of synthesis. However, amorphization of QCs remains limited to this Li-bearing compositions, such as i-Al60Li30Cu10 and crystalline Al5Li3Cu (Winters and Hammack 1993).
The AlCuZ (Z = Fe, Ru, V, Cr, and Mn; see Table 1) QCs are all characterized by icosahedral symmetry with bulk modulus comprised between 79 and 155 GPa, as determined using the DAC either with the conventional powder XRD (Sadoc et al. 1998) or in situ energy-dispersive XRD (Ponkratz et al. 2001) up to about 36 GPa, and Si oil and methanol–ethanol employed as pressure media, respectively. For comparison, the EoS of the Z elements (Anzellini et al. 2019, 2022 for Ru and Cr; Ding et al. 2007 for V; Fujihisa and Takemura 1996 for Mn) are shown in Fig. 1c along with the compressibility of i-Al65Cu20Ru15, i-Al62Cu15.5Cr12.5, i- Al62Cu15.5Mn12.5, and i-Al62Cu15.5V12.5. Once again, the compressibility of the i-QCs appears mostly influenced by the elastic behavior of the pure elements. An exception is represented by i-Al65Cu20Ru15 and i-Al62Cu15.5Cr12.5 as consequence of the high K0 of the latter (see Table 1). This ternary system also includes the composition of icosahedrite. Experiments at high P on Al–Cu–Fe QCs were reported by Chen et al. (1991), who proved the stability of the QC phase.
The finding of icosahedrite (Bindi et al. 2009) has been of inspiration for the experimental studies conducted on synthetic i-Al63Cu24Fe13 at high pressure using in situ angle-dispersive X-ray (ADXD) assisted by synchrotron radiation (Stagno et al. 2015). These experiments differ from what reported previously in both the higher brilliance of the synchrotron radiation, that allows a better resolution at HP within a wide d-spacing, and the choice of Ne as pressure medium rather than the less hydrostatic Si oil or methanol + ethanol ± water (Jayaraman 1983). In addition, seven out of eight interplanar distances have been examined including the peaks (12,16) at 5.53 Å and (8,4) at 8.94 Å that are shown to be more compressible over the P range of investigation up to 76 GPa (Stagno et al. 2021) both in compression and decompression. The estimated reduction of the lattice parameter from the ambient pressure value of 12.64 Å is by ~ 10%. The compressional data on i-Al63Cu24Fe13 fitted to a Birch-Murnaghan EoS do not show a significant change of the bulk modulus of 121(8) GPa (\({K}_{0}{\prime}\) of 3.68 ± 4) at 76 GPa with respect to the 113.7 GPa (\({K}_{0}{\prime}\) of 4.22) obtained for the same i-QC compressed to 50 GPa (Stagno et al. 2015). A fit to a unique EoS results to a K0 of 120(2) GPa and \({K}_{0}{\prime}\) of 3.87(14). These EoS parameters are slightly smaller than 139 GPa (\({K}_{0}{\prime}\) of 2.7) for i-Al62Cu25.5Fe12.5 proposed by Sadoc et al. (1994). Figure 1d shows the variation of the volume V/V0 as function of P for the compositions displayed in Table 1 extrapolated to the same P by assuming neither phase transitions nor decompositions for experimental data collected by Takagi et al. (2015) and refitted by Stagno et al. (2021). Such assumption is supported by the lack of evidence of either P-induced structural phase transitions or amorphization in the literature. Further, the fit of integrated data collected up to 104 GPa produces a bulk modulus of 117(3) GPa (\({K}_{0}{\prime}\) of 4.45 ± 15), which is again consistent with the previous fit by Stagno et al. (2015). On the other hand, the extrapolated V/V0 for the rhombohedral and pentagonal approximant phases have the same physical behavior at high P, as reflected by the same EoS parameters (Lefebvre et al. 1995).
Unfortunately, no experimental data exist to date to test the stability of decagonal Al71Ni24Fe5 (the analogue of decagonite; Bindi et al. 2015a, b) at high P. The only available data are from Lefebvre et al. (1995) and (Hasegawa et al. 1999a, b) referred to decagonal Al72Ni20Co8 for which a bulk modulus of 155 GPa \({(K}_{0}{\prime}\) of 2.0) and 120 GPa (\({K}_{0}{\prime}\) of 5) was proposed, respectively. Additional compositions are reported in Table 1 such as those in the AlPdMn and AlPdRe. AlPdMn was investigated up to 70 GPa in the absence of pressure medium (Hasegawa et al. 1999a) with a quite low bulk modulus (100 GPa and \({K}_{0}{\prime}\) of 5.3). AlPdRe was compressed up to about 36 GPa in Si oil (Sadoc et al. 1998) for which the high bulk modulus of 180 GPa has been reported.
3 Al-based quasicrystals at high temperatures
The investigation of the behavior of QCs at high T has been classically motivated by the question whether QCs are stabilized by energy or entropy. When scaled to natural scenarios, the fact that QCs can retain their structure to high T would have dramatic implications for their possible occurrence in igneous rocks as well as in post-impact thermal cooling processes proving their stability upon slow cooling over the geological timescale. The discovery of natural QCs opened a scenario where these minerals can play a role in crystallization mechanisms driven by high P and T conditions. Synthetically produced QCs are known to form by rapid quench from a liquid alloy, as reported by early studies by Shechtman et al. (1984) and by Ball and Lloyd (1985), who faced the issue whether the quasiperiodic structure could be intermediate between amorphous and crystalline. In fact, early HT investigations of QCs seemed to point out that these were metastable phases. At least, this was the case of i-Al86Mn14 that, once annealed at T slightly less than 400 °C for 10 min, transformed to orthorhombic Al6Mn, the stable phase (as reported by Chen et al. 1985), with some relics of icosahedral QC, the metastable phase. An important step forward for the same QC alloy composition was represented by the observation of congruent melting induced by electron beam irradiation (Knapp and Follstaedt 1987) of both icosahedral and decagonal Al85.2–79.5Mn14.8–20.5, with the melting T of the latter (i.e., 965 ± 20 °C) being higher than that of the icosahedral QC (i.e., 910 ± 20 °C) but both lower than the melting T of the corresponding crystalline analogues. The same year, the solidification of an icosahedral QC with composition Al65Cu20Fe15 was described by Tsai et al. (1987) to be exceptionally stable after annealing for 48 h at 845 °C. The year after, the structural stability of icosahedral Al65Cu20Ru15 was reported along with its stability after annealing for 12 h at ~ 860 °C (Tsai et al. 1988). These observations were followed by the discovery of single decagonal QCs with compositions of Al75Ni9-16Fe9-21 and Al75Ni9-16Co9-21 (Tsai et al. 1989) for which a low thermal stability was later reported to be between 847 and 930 °C (Grushko et al. 1996). On the contrary, a similar d-QC with composition Al65Cu15Co20 was shown to be stable up to 940 °C by thermal annealing but decomposed to a cubic fcc phase when treated with electron beam irradiation (He et al. 1991). These studies show, therefore, a common protocol consisting of quenching the QC from a not well constrained high T owing to the use of arc furnaces followed by the verification of the structural stability upon annealing as a function of T and time.
The Al–Cu–Fe system was investigated in detail at ambient P by Bancel (1999), who showed the coexistence of the icosahedral QC along with crystalline phases, such as β, λ, and ω (already described by Bradley and Goldschmidt 1939) all surrounded the icosahedral region with a narrow stability field. This field ranges Cu–Fe content from 24–12 to 26–13 atom% within ~ 100 °C up to ~ 900 °C, beyond which the alloy was proposed to melt incongruently to produce a liquid coexisting with a cubic and/or monoclinic crystalline phases and then being totally molten at ~ 1000 °C (Gratias et al. 1993; Bancel 1999). The relatively low melting point of the i-QC reflects the high Al content, the melting temperature of which is about 660 °C and ambient P. The thermal stability of the ternary Al–Cu–Fe QC allowed to study the lattice parameter a6D to be investigated as a function of T leading, therefore, to the determination of the thermal expansion coefficient of 12.3·10–6·K−1 (Quivy et al. 1994).
Zhang and Lück (2003a) and Zhang et al. (2005) performed phase equilibria studies of metallurgic interest within the Al–Cu–Fe system between 560 and 900 °C at ambient P under a controlled inert atmosphere. These studies reported 27 solidification reactions from which icosahedral QC never crystallized as liquidus phase except in the case of Al62Cu34.5Fe3.5 melt composition that, after a cooling process, resulted in the formation of the icosahedral QC with composition Al64.2Cu25.7Fe10.1.
Zhang and Lück (2003b) also described the solidification sequence for 31 starting compositions in the vicinity of icosahedrite through experiments performed at ambient pressure and temperatures above 1000 °C. Among all these compositions, Al64Cu23Fe13, Al65Cu25Fe10, and Al67Cu21.5Fe11.5 are those recalling the composition of icosahedrite. The solidification sequence at 1 atm for these three compositions as function of temperature (although not always clearly stated) is:
-
1.
Liquid (Al64Cu23Fe13) → liquid + λ-phase (Al74.7Cu3.9Fe21.4) → liquid + λ-phase + i-QC-phase (Al63.6Cu23.8Fe12.6) → λ-phase + i-QC-phase (Al63.6Cu23.8Fe12.6);
-
2.
Liquid (Al65Cu25Fe10) → liquid + λ-phase (Al74.7Cu3.8Fe21.8) at 986 °C → liquid + λ-phase + i-QC-phase (Al65.4Cu25.5Fe12.1) at 818 °C → liquid + λ-phase + i-QC-phase + β-phase (Al56.4Cu40.4Fe3.2) at 671 °C → λ-phase + i-QC-phase + β-phase + η-phase (Al49.7Cu49.7Fe0.6) + θ-phase (Al68.1Cu31.7Fe0.2) at 576 °C;
-
3.
Liquid (Al67Cu21.5Fe11.5) → liquid + λ-phase (Al74.5Cu3.9Fe21.6) → liquid + λ-phase + i-QC-phase (Al66.4Cu21.4Fe12.2) → liquid + λ-phase + i-QC-phase + β-phase (Al56.9Cu40.9Fe2.2) → λ-phase + i-QC-phase + β-phase + θ-phase (Al67.6Cu32.1Fe0.3).
These phase equilibria studies and solidification paths, although delivered to the metallurgy community, have represented a useful guide to the interpretation of naturally occurring QCs as well as the run products of HP and T experiments.
It is important to remember that i-QCs in nature appear to retain their structure even after experiencing exposure to the highly oxidizing terrestrial atmosphere after their fall on Earth. Because of the high Al content along with other O2-sensitive elements like Cu and Fe, the i-QC can be reasonably considered highly exposed to oxidation. Explorative and preliminary experiments were performed at T between 700 and 1200 °C and ambient P (Table 2) using a synthetic icosahedral quasicrystalline powder as starting material with nominal composition of Al65Cu23Fe12 (purity ≥ 99.9%, Sigma-Aldrich) with negligible amounts of AlFe3 resulting from the industrial synthesis preparation. The morphology of the starting material is shown in Fig. 2a along with the typical XRD pattern (Fig. 2b) and the forbidden QC rotational symmetry (Fig. 2c). Experiments were performed using a Deltech furnace and the starting powder loaded into a Pt capsule opened on the top side and placed in the hot-spot portion of the furnace. The temperature during the experiments was monitored with a Pt/Pt-10%Rh (S-type) thermocouple inserted within an alumina sleeve positioned near the top of the capsule. The samples were kept at constant target temperature for a period of 1 and 6 h. Each run was quenched by dropping the capsule into liquid nitrogen. The recovered run products (Fig. 3a–f) along with the X-ray diffraction patterns in Fig. 4 reveal a gradual exsolution of an alumina-rich layer that rims the icosahedrite particles. Powder X-ray diffraction data confirm that the structure of the starting material is preserved with the increase of temperature well above that proposed by Bancel (1999). These results shed light on the oxidation of synthetic icosahedrite consisting in the formation of refractory Al2O3. Further experiments consisting of heat treatment at variable oxygen fugacity would be ideal to explore the O-dependence on the formation of additional phases and the effect on the melting T.
a–f Images of the run products listed in Table 2 taken with the secondary electrons suggest the exsolution starting at 900 °C of an alumina-rich layer that rims the icosahedrite particles
4 Quasicrystals at high temperature and pressure
Despite the important role that T might have on structural changes of QCs and their physical properties, to date, the investigation of the stability of QCs at simultaneous HP and HT has been very limited. Both natural Al63Cu24Fe13 icosahedrite (Bindi et al. 2009) and Al71Ni24Fe5 decagonite (Bindi et al. 2015a, b) were predicted by high-temperature studies within the Al–Cu–Fe and Al–Ni–Fe system (Bancel 1999; Tsai et al. 1989; Lemmerz et al. 1994). In addition, these natural QCs are accepted to have experienced HP during shock-impact events.
A few studies describe the synthesis and stability of i-QC at relatively low temperature (350–840 °C) and low pressure (uniaxial compression testing; Kang and Dubois 1992) up to 1.64 GPa (Turquier et al. 2004), which cannot be directly applied to explain the occurrence of icosahedrite in the Khatyrka meteorite where typical HP-T minerals (i.e., stishovite and ahrensite) were found. A first indication that comes from the observation of natural QCs is their capacity to retain the icosahedral and decagonal structure at extreme conditions. Recent experimental simulations allowed to understand whether i-AlCuFe retains icosahedral symmetry at high P and T or, rather, it transforms either into a periodic approximant (Lefebvre et al. 1995; Quiquandon et al. 1996) or other crystalline phases (Gratias et al. 1993; Barbier et al. 1993). Subsequent experimental HP-T data conducted using both the multi-anvil apparatus and the diamond anvil cell either ex situ or in combination with synchrotron radiation allowed to monitor the structural evolution during compression/heating in real time. Stagno et al. (2014) reported the first experiment at hydrostatic P of 5 GPa and T up to 1500 °C using the multi-anvil apparatus combined with the in situ synchrotron radiation at Spring8 (Japan). The starting material used in this study was the synthetic quasicrystalline powder mentioned above with variable grain size ≤ 25 μm and composition of Al65Cu23Fe12, quite similar to icosahedrite (Al63Cu24Fe13), and both falling into the stability field of the icosahedral phase reported by Bancel (1999). Their results, supported by X-ray diffraction (in energy-dispersive mode and single-crystal XRD on the recovered QC sample), chemical and textural analyses (suggesting the role of P in increasing the melting T with no approximants formed) provided evidence of the structural stability of synthetic icosahedrite at 5 GPa up to 1400 °C. In addition, the obtained thermal expansion coefficients at 5 GPa of 4.67·10−5A°−1·K−1 and 5.22·10−8A−1·K−1, resulted only slightly different than those determined by Quivy et al. (1994) at ambient P.
A further important achievement is represented by the investigation of synthetic icosahedrite at simultaneous HP and T using the diamond anvil cell combined with a double-sided laser heating system assisted by synchrotron radiation at HPCAT and GSECARS beamlines of Argonne National Laboratory (Chicago, US) described by Stagno et al. (2015). In these experiments, performed using Ne as pressure medium and angle-dispersive X-ray diffraction, the synthetic quasicrystalline powder was first compressed to ~ 42 GPa and then heated to ~ 1560 °C while collecting X-ray diffraction patterns to monitor any possible transformation or melting. The sample was then cooled down to 730 °C before being quenched. Stagno et al. (2015) reported neither amorphization nor phase transformation, rather the presence of the characteristic diffraction peaks including those at low theta-angles useful to calculate the six-dimensional lattice parameter (Bindi et al. 2011). The same result was observed after heating up to ~ 1837 °C and then quenched directly to room-temperature. The confirmation that the i-QC survived to such HP and T was given by the evidence of the fivefold symmetry obtained by ex situ single-crystal X-ray diffraction measurements on a micrometer-sized grain handpicked from the open cell.
A more extensive study is represented by the investigation of the phase equilibria within the Al–Cu–Fe system where synthetic Al65Cu23Fe12 was used as starting material for experiments performed using the multi-anvil press within a P range of 3, 5, and 21 GPa and T from 800 °C to 1700 °C. Here, the results can be summarized with the i-QC being stable at 3 GPa from 800 °C to 1100 °C at least in equilibrium with quenched phases that resemble those found in Khatyrka like (Fe-bearing) stolperite, cupalite, and khatyrkite ± a melt. At 5 GPa, the stability of icosahedrite is confirmed at 1200 °C along with the same additional Al-bearing phases, although we cannot exclude that i-QC could be stable at 1200 °C ≤ T < 1500 °C. At 21 GPa, the quasicrystal was recovered from runs up to 1500 °C along with the presence of both Al-bearing and liquid phases. Some important aspects are necessary to point out as the following: (1) the successful synthesis of an icosahedral QC phase at 5 GPa and 1200 °C starting from a mixture of pure Al, Cu, and Fe in relative ratios typical of icosahedrite (run PR1205r); (2) the observation of two icosahedral QCs very similar in composition (runs PR1197 and PR1205r) that matches with the occurrence of the two icosahedral QCs reported by Bindi et al. (2016) as Al62.64Cu24.11Fe13.26 and Al65Cu20.81Fe14.19; (3) pressure acts to stabilize the QC structure to higher T as indicated by the positive Clapeyron–Clausius slope in Fig. 6 of Stagno et al. (2017).
Another important achievement is represented by a revision of the solidification sequence proposed by Zhang and Lück (2003b). Stagno et al. (2017) proposed the following reactions sequence from 1400 to 800 °C at 3 GPa:
Liquid (Al65Cu23Fe12) [T > 1400 °C] → (unconstrained) → Liq + Fe-khatyrkite (Al65.6Cu19Fe15.4) + stolperite (Al48.8Cu45.9Fe5.36) + cupalite (Al43.9Cu54.7Fe1.4) at 1400 °C → Liq + Fe-khatyrkite (Al66.6Cu17.7Fe15.7) + stolperite (Al49.9Cu44.1Fe6.0) at 1200 °C → Liq + Fe-khatyrkite (Al66.6Cu17.8Fe15.6) + stolperite (Al52.2Cu40.1Fe7.7) + icosahedrite (Al60.6/62.2Cu26.2/21.9Fe13.2/15.9) at 1100 °C → stolperite (Al53.8Cu40.2Fe6.0) + icosahedrite (Al66.5Cu20.2Fe13.3) at 800 °C.
The proposed reaction sequence from 1500 to 1200 °C at 5 GPa is:
Liquid (Al65Cu23Fe12) [T > 1500 °C] → (unconstrained) → Liq + Fe-khatyrkite (Al65.2Cu19.5Fe15.3) + stolperite (Al48.2Cu45.5Fe6.4) at 1400 °C → stolperite (Al49.7Cu44.2Fe6.1) + icosahedrite (Al65/62.6Cu20.8/24.1Fe14.2/13.3) [+ cupalite (Al44.5Cu53.9Fe1.6) + Fe-khatyrkite (Al67.2Cu9.6Fe23.2)] at 1200 °C.
Finally, the proposed reaction sequence from 1700 to 1400 ˚C at 21 GPa is:
Liquid (Al65Cu23Fe12) at T > 1700 °C → Liq (Al12.2Cu21.6Fe66.2) + Al at 1700 °C → Liq + Fe-rich cupalite (Al48.8Cu36.1Fe15.1) + icosahedrite (Al64.7Cu20.2Fe15) + aluminum [Al(Cu,Fe)] at 1500 °C → stolperite (Al52.8Cu31.7Fe15.3) + icosahedrite (Al68.2Cu22.4Fe9.4) at 1450 °C → icosahedrite (Al65.5Cu23.3Fe11.2) at 1450 > T > 1400 °C (7).
It must be emphasized that the experimental studies producing icosahedrite performed by multi-anvil and DAC lasted from 5 to 300 min at high P and T implying, therefore, the kinetics stability of the QC structure. This interval is well beyond that applied in experiments where the QC was either annealed or irradiated by an electron beam. Dynamic experiments certainly help to reproduce mechanism like shock impact at the origin of QC formation; however, static experiments carried out using the diamond anvil cell and multi-anvil presses are necessary to monitor structural changes under HP-T, breakdown reactions, and melting relations at HP-T. Considering the possible origin of QCs in hypervelocity impacts in outer space, both i-AlCuFe quasicrystal and d-QC were reported as run products of shock experiments during which shock pressures up to 35 GPa were generated at T of 400 °C within less than 1 µs (Asimow et al. 2016; Oppenheim et al. 2017a, b; Hu et al. 2020). Within such a short time, both the textural contacts between the quasicrystalline phases and the Al–Cu–Fe alloys as well as their chemical compositions were reproduced as occurring in the Khatyrka meteorite. In addition, the recovered Al–Cu–Fe QCs hosted additional elements like Cr and Ni, that represents important evidence of the stability of QCs even beyond the compositional field previously proposed by Bancel (1999).
5 Further remarks
If, on one side, the last 4 decades have seen a slightly decreased interest for the study of QCs in terms of synthesis within various systems and their possible applications (Steurer 2018; Dubois 2023), the investigation of the structural stability of these materials as function of P and T remains limited to the studies reported in Table 1. From the geoscience perspective, the finding of decagonite (Al71Ni24Fe5; Bindi et al. 2015a, b; Buganski and Bindi 2021), a second icosahedral quasicrystal (Al62Cu31.2Fe6.8; Bindi et al. 2016) and proxidecagonite, Al34Ni9Fe2, the first natural known periodic crystalline approximant to decagonite (Al71Ni24Fe5; Bindi et al. 2018), all would benefit from high P studies to determine their elastic parameters as well as their behavior when compressed and heated. Similar investigations should be undertaken on those minerals reported to coexist with the natural QCs like stolperite, hollisterite, kryachkoite, cupalite, and khatyrkite. These studies would be a guide to accurately determine the P–T conditions for the stability of QCs in nature and the mechanisms for their formation. Afterall, the presence of QCs has been also proposed in more common scenarios like sedimentary rocks as consequence of the recrystallization when clay and salt react (Kharitonova et al. 2015).
Additional open questions remain on the physical and chemical properties of the liquid coexisting with the QCs or from which QCs form. Are the interatomic distances retained from solid QCs to liquids and back? Such an answer would help to understand whether QCs might have formed from a liquid alloy that migrated to the center of a planetesimal to form its core.
Data availability
Data are available
References
Akahama Y, Mori Y, Kobayashi M, Kawamura H, Kimura K, Takeuchi S (1989) Pressure induced amorphization of quasi crystals. J Phys Soc Jpn 58:2231–2234
Akahama Y, Mori Y, Kobayashi M, Kawamura H, Kimura K, Takeuchi S (1991) Pressure-induced phase transformation of quasicrystals. J Phys Soc Jpn 60:1988–1993
Amazit Y, Perrin B, Fischer M, Itie JP, Polian A (1997) X-ray diffraction measurements in icosahedral Al–Pd–Mn up to 40 GPa. Phil Mag A75:1677–1688
Anzellini S, Errandonea D, Cazorla C, MacLeod S, Monteseguro V, Boccato S, Bandiello E, Diaz Anichtchenko D, Popescu C, Beavers CM (2019) Thermal equation of state of ruthenium characterized by resistively heated diamond anvil cell. Sci Rep 9:14459
Anzellini S, Errandonea D, Burakovsky L, Proctor JE, Turnbull R, Beavers CM (2022) Characterization of the high-pressure and high-temperature phase diagram and equation of state of chromium. Sci Rep 12:6727
Asimow PD, Lin C, Bindi L, Ma C, Tschauner O, Hollister LS, Steinhardt PJ (2016) Shock synthesis of quasicrystals with implications for their origin in asteroid collisions. Proc Nat Acad Sci 113:7077–7081
Ball MD, Lloyd DJ (1985) Particles apparently exhibiting five-fold symmetry in Al-Li-Cu-Mg alloys. Scr Metall 19:1065–1068
Bancel PA (1999) Order and disorder in icosahedral alloys. Quasicrystals, 2nd edn. World Scientific, Cham, pp 17–55
Barbier J-N, Tamura N, Verger-Gaugry J-L (1993) Monoclinic Al13Fe4 approximant phase: a link between icosahedral and decagonal phases. J Non-Cryst Solids 153–154:126–131
Bindi L, Parisi G (2023) Quasicrystals: fragments of history and future outlooks. Rend Fis Acc Lincei 34:317–320
Bindi L, Steinhardt PJ, Yao N, Lu PJ (2009) Natural quasicrystals. Science 324:1306–1309
Bindi L, Steinhardt PJ, Yao N, Lu PJ (2011) Icosahedrite, Al63Cu24Fe13, the first natural quasicrystal. Am Miner 96:928–931
Bindi L, Eiler JM, Guan Y, Hollister LS, MacPherson G, Steinhardt PJ, Yao N (2012) Evidence for the extraterrestrial origin of a natural quasicrystal. Proc Nat Acad Sci 109:1396–1401
Bindi L, Yao N, Lin C, Hollister LS, Andronicos CL, Distler VV, Eddy MP, Kostin A, Kryachko V, MacPherson GJ, Steinhardt WM, Yudovskaya M, Steinhardt PJ (2015a) Natural quasicrystal with decagonal symmetry. Sci Rep 5:9111
Bindi L, Yao N, Lin C, Hollister LS, Andronicos CL, Distler VV, Eddy MP, Kostin A, Kryachko V, MacPherson GJ, Steinhardt WM, Yudovskaya M, Steinhardt PJ (2015b) Decagonite, Al71Ni24Fe5, a quasicrystal with decagonal symmetry from the Khatyrka CV3 carbonaceous chondrite. Am Miner 100:2340–2343
Bindi L, Lin C, Ma C, Steinhardt PJ (2016) Collisions in outer space produced an icosahedral phase in the Khatyrka meteorite never observed previously in the laboratory. Sci Rep 6:38117
Bindi L, Pham J, Steinhardt PJ (2018) Previously unknown quasicrystal periodic approximant found in space. Sci Rep 8:16271
Bradley AJ, Goldschmidt HJ (1939) An X-ray study of slowly cooled iron-copper-aluminium alloys. Mon J Inst Metals 6:157–210
Buganski I, Bindi L (2021) Insight into the structure of decagonite – the extraterrestrial decagonal quasicrystal. IUCrJ 8:87–101
Chen HS, Chen CH, Inoue A, Krause JT (1985) Density, Youngs modulus, specific heat, and stability of icosahedral Al86Mn14. Phys Rev B 32(4):1940–1944
Chen ZH, Jiang XY, Wan Y, Zhou DS, Huang PY (1991) Superhigh pressure consolidation of Al-based alloy quasicrystalline powder. Scr Metall Mater 25:159
Decremps F, Gauthier M, Ricquebourg F (2006) Accurate equation of state of AlPdMn up to 35 GPa and pressure effect on the frozen-in Phason strain. Phys Rev Lett 96:105501
Dewaele A, Loubeyre P, Mezouar M (2004) Equations of state of six metals above 94 GPa. Phys Rev B 70:094112
Ding Y, Ahuja R, Shu J, Chow P, Luo W, Mao H-K (2007) Structural phase transition of vanadium at 69 GPa. Phys Rev Lett 98:085502
Dubois J-M (2000) New prospects from potential applications of quasicrystalline materials. Mater Sci Eng A294–296:4–9
Dubois J-M (2012) Properties and applications of quasicrystals and complex metallic alloys. Chem Soc Rev 41:6760–6777
Dubois J-M (2023) Potential and marketed applications of quasicrystalline alloys at room temperature or above. Rend Fis Acc Lincei. https://doi.org/10.1007/s12210-023-01170-4
Fujihisa H, Takemura K (1995) Stability and the equation of state of α-manganese under ultrahigh pressure. Phys Rev B 52:13257
Fujihisa H, Takemura K (1996) Equation of state of cobalt up to 79 GPa. Phys Rev B 54:5
Gratias D, Calvayrac Y, Devaud-Rzepski J, Faudot F, Harmelin M, Quivy A, Bancel PA (1993) The phase diagram and structures of the ternary AlCuFe system in the vicinity of the icosahedral region. J Non-Cryst Solids 153–154:482–488
Grushko B, Holland-Moritz D, Bickmann K (1996) Decagonal quasicrystals in Al Co and ternary alloys containing Cu and Ni. J Alloy Compd 236(1–2):243–252
Hanfland M, Loa I, Syassen K, Schwarz U, Takemura K (1999) Equation of state of lithium to 21 GPa. Solid State Commun 112(3):123–127
Hasegawa M, Tsai AP, Yagi T (1999a) Stability and strain of decagonal Al-Ni-Co quasicrystal under high pressure up to 70 GPa. Phil Mag Lett 79:691–698
Hasegawa M, Tsai AP, Kondo T, Yagi T, Kikegawa T (1999b) In situ X-ray powder diffraction study on stability of icosahedral Al–Pd–Mn high quality single quasi-crystal under high pressure up to 70 GPa. J Non-Cryst Solids 250–252(2):849–854
He Y, Chen H, Meng XF, Poon SJ, Shiflet GJ (1991) Stability investigation of a decagonal Al–Cu–Co quasicrystal. Philos Mag Lett 63(4):211–216
Hollister LS, Bindi L, Yao N, Poirier GR, Andronicos CL, MacPherson GJ, Lin C, Distler VV, Eddy MP, Kostin A, Steinhardt WM, Yudovskaya M, Eiler JM, Guan Y, Clarke JJ, Steinhardt PJ (2014) Impact-induced shock and the formation of natural quasicrystals in the early solar system. Nat Commun 5:3040
Hu J, Asimow PD, Ma C, Bindi L (2020) First synthesis of a unique icosahedral phase from the Khatyrka meteorite by shock recovery experiment. IUCrJ 7:434–444
Jaya NV, Yousuf M, Ragunathan VS, Natarajan S (1987) High-pressure X-ray studies of quasicrystal Al-18 at. % Fe. J Phys F 17:225
Jayaraman A (1983) Diamond anvil cell and high-pressure physical investigations. Rev Mod Phys 55:65
Johnson E, Staun Olsen J, Wood JV, Gerward L (1988) High pressure structural study of quasicrystalline AlMn. Mat Sci Eng 99:403–406
Kang SS, Dubois JM (1992) Pressure-induced phase transitions in quasi-crystals and related compounds. Europhy Lett 18:45–51
Kharitonova GV, Shein EV, Pugachevskii MA, Komarova VS, Tyugai ZN, Manucharov AS, Ri TD (2015) Clay-salt soil formations in situ and ex situ. Moscow Univ Soil Sci Bull 70:50–57
Knapp JA, Follstaedt DM (1987) Measurements of melting temperatures of quasicrystalline Al–Mn phases. Phys Rev Lett 58:2454
Krauss G, Steurer W (2004) Why study quasicrystals at high pressures? In: Katrusiak A (ed) High-pressure crystallography. McMillan, pp 521–526
Kuwayama Y (2008) Ultrahigh pressure and high temperature experiments using a laser heated diamond anvil cell in multimegabar pressures region. Rev High Press Sci Technol 18:3–10
Lefebvre S, Bessièref M, Calvayrac Y, Ltié JP, Polian A, Sadoc A (1995) Stability of icosahedral Al–Cu–Fe and two approximant phases under high pressure up to 35 GPa. Philos Mag B 72:101–113
Lemmerz U, Grushko B, Freiburg C, Jansen M (1994) Study of decagonal quasicrystalline phase formation in the Al–Ni–Fe alloy system. Phil Mag Lett 69:141–146
Levine D, Steinhardt PJ (1984) Quasicrystal: a new class of ordered structures. Phys Rev Lett 53:2477–2480
Lin C, Hollister LS, MacPherson GJ, Bindi L, Ma C, Andronicos CL, Steinhardt PJ (2017) Evidence of cross-cutting and redox reaction in Khatyrka meteorite reveals metallic-Al minerals formed in outer space. Sci Rep 7:1637
Ma C, Tschauner O, Beckett JR, Liu Y, Rossman GR, Sinogeikin SV, Smith JS, Taylor LA (2016) Ahrensite, c-Fe2SiO4, a new shock-metamorphic mineral from the Tissint meteorite: Implications for the Tissint shock event on Mars. Geochim Cosmochim Acta 184:240–256
Ma C, Lin C, Bindi L, Steinhardt PJ (2017) Hollisterite (Al3Fe), kryachkoite (Al, Cu)6(Fe, Cu), and stolperite (AlCu): three new minerals from the Khatyrka CV3 carbonaceous chondrite. Am Miner 102:690–693
MacPherson GJ, Andronicos CL, Bindi L, Distler VV, Eddy MP, Eiler JM, Guan Y, Hollister LS, Kostin A, Kryachko V, Steinhardt WM, Yudovskaya M, Steinhardt PJ (2013) Khatyrka, a new CV3 find from the Koryak Mountains, Eastern Russia. Meteorit Planet Sci 48:1499–1514
Oppenheim J, Ma C, Hu J, Bindi L, Steinhardt PJ, Asimow PD (2017a) Shock synthesis of five-component icosahedral quasicrystals. Sci Rep 7:15629
Oppenheim J, Ma C, Hu J, Bindi L, Steinhardt PJ, Asimow PD (2017b) Shock synthesis of decagonal quasicrystals. Sci Rep 7:15628
Parthasarathy G, Gopal ESR, Krishnamurthy HR, Pandit R, Sekhar JA (1986) Quasi crystalline Al–Mn alloys: pressure induced crystallization and structural studies. Curr Sci 55:517–520
Ponkratz U, Nicula R, Jianu A, Burkel E (2001) In situ high-pressure X-ray diffraction study of icosahedral Al–Cu–TM (TM = V, Cr, Mn) alloys. J Phys 13:549
Presnall DC (1995) Phase diagrams of Earth-forming minerals. Mineral physics and crystallography, a handbook of physical constants by Ahrens T. J. American Geophysical Union, Washington, DC, pp 248–268
Quiquandon M, Quivy A, Devaud J, Faudot F, Lefebvre S, Bessière M, Calvayrac Y (1996) Quasicrystal and approximant structures in the Al–Cu–Fe system. J Phys 8:2487–2512
Quivy A, Lefebvre S, Soubeyroux JL, Filhol A, Bellissent R, Ibberson RM (1994) High-resolution time-of-flight measurements of the lattice parameter and thermal expansion of the icosahedral phase Al62Cu25.5Fe12.5. J Appl Crystallogr 27:1010–1014
Sadoc A, Itié JP, Polian A, Lefebvre S, Bessière A, Calvayrac Y (1994) X-ray absorption and diffraction spectroscopy of icosahedral Al–Cu–Fe quasicrystals under high pressure. Phil Mag 70:855–866
Sadoc A, Itié JP, Polian A, Berger C, Poon SJ (1998) High-pressure X-ray diffraction of icosahedral Al–Cu–Ru and Al–Pd–Re quasicrystals. Phil Mag A77:115–128
Sato-Sorensen Y, Sorensen LB (1989) Compressibility and stability of icosahedral AlMn up to 28 GPa. Phys Rev B 39:2654–2660
Shechtman D, Blech I, Gratias D, Cahn J (1984) Metallic phase with long-range orientational order and no translational symmetry. Phys Rev Lett 53:1951–1954
Stagno V, Bindi L, Shibazaki Y, Tange Y, Higo Y, Mao H-K, Steinhardt PJ, Fei Y (2014) Icosahedral AlCuFe quasicrystal at high pressure and temperature and its implications for the stability of icosahedrite. Sci Rep 4:5869
Stagno V, Bindi L, Park C, Tkachev S, Prakapenka VB, Mao H-K, Hemley RJ, Steinhardt PJ, Fei Y (2015) Quasicrystals at extreme conditions: the role of pressure in stabilizing icosahedral Al63Cu24Fe13 at high temperature. Am Miner 100:2412–2418
Stagno V, Bindi L, Steinhardt PJ, Fei Y (2017) Phase equilibria in the nominally Al65Cu23Fe12 system at 3, 5 and 21 GPa: implications for the quasicrystal-bearing Khatyrka meteorite. Phys Earth Plan Inter 271:47–56
Stagno V, Bindi L, Takagi S, Kyono A (2021) Can quasicrystals survive in planetary collisions? Progr Earth Plan Sci 8:27
Steurer W (2018) Quasicrystals: What do we know? What do we want to know? What can we know? Acta Crystallogr Sect A Found Adv 74(1):1–11
Swamy V, Saxena SK, Sundman B, Zhang J (1994) A thermodynamic assessment of silica phase diagram. J Geophys Res 99:787–794
Takagi S, Kyono A, Mitani S, Sugano N, Nakamoto Y, Hirao N (2015) X-ray diffraction study of the icosahedral AlCuFe quasicrystal at megabar pressures. Mat Lett 161:13–16
Tsai A, Inoue A, Masumoto T (1987) A stable quasicrystal in Al–Cu–Fe system. Jpn J Appl Phys 26:L1505–L1507
Tsai A, Inoue A, Masumoto T (1988) New stable icosahedral in Al–Cu–Ru and Al–Cu–Os Alloys. Jpn J Appl Phys 9:L1587–L2190
Tsai A, Inoue A, Masumoto T (1989) A new decagonal Al–Ni–Fe and Al–Ni–Co alloys prepared by liquid quenching. Mater Trans JIM 30:150–154
Turquier F, Cojocaru VD, Stir M, Nicula R, Lathe C, Burkel E (2004) Formation and stability of single-phase Al–Cu–Fe quasicrystals under pressure. Rev Adv Mat Sci 8:147–151
Winters RR, Hammack WS (1993) Pressure-induced amorphization of R-Al5Li3Cu: a structural relation among amorphous metals, quasi-crystals, and curved space. Science 260:202–204
Yagi T, Akaogi M, Shimomura O, Suzuki T, Akimoto S (1987) In situ observation of the olivine-spinel transformation in Fe2SiO4 using synchrotron radiation. J Geophys Res 92:6207–6213
Zhang L, Lück R (2003a) Phase diagram of the Al–Cu–Fe quasicrystal-forming alloy system. I. Liquidus surface and phase equilibria with liquid. Z Metallkd 94:91–97
Zhang L, Lück R (2003b) Phase diagram of the Al-Cu-Fe quasicrystal-forming alloy system. V. Solidification behavior of Al-Cu-Fe quasicrystal forming alloys. Z Metallkd 94:774–781
Zhang L, Schneider J, Reinhard L (2005) Phase transformations and phase stability of the AlCuFe alloys with low-Fe content. Intermetallics 13:1195–1206
Zhou L, Che R, Zhang D, Xie L (1996) High pressure X-ray diffraction of AlNiCo using synchrotron radiation. In: Janot C, Mosseri R (eds) Proceeding of the fifth international conference on quasicrystals, Avignon May 1995. World Scientific, Singapore, p 192
Acknowledgements
V.S. is grateful to financial support by Sapienza University through “Bandi di Ateneo 2021” and to collaborators from Carnegie Institution of Washington and HPSTAR (Beijing) for fruitful discussion and providing access to HP-T facilities.
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to the topical collection “Quasicrystals: State of the art and outlooks” originated from an international conference organized by the Accademia dei Lincei, held in Rome on November 18, 2022 in the frame of the 2022 International Year of Mineralogy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stagno, V., Bindi, L. Quasicrystals at high pressures and temperatures: a review. Rend. Fis. Acc. Lincei 34, 727–738 (2023). https://doi.org/10.1007/s12210-023-01183-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-023-01183-z