Abstract
The metabolic theory of ecology (MTE) has explained the taxonomic richness of ectothermic species as an inverse function of habitat mean temperature. Extending this theory, we show that yearly temperature cycles reduce metabolic rates of taxa having short generation times. This reduction is due to Jensen’s inequality, which results from a nonlinear dependency of metabolic rate of organisms on temperature. It leads to a prediction that relatively lower species richness is found in habitats with larger amplitudes of yearly temperature cycles where mean temperatures and other conditions are similar. We show that metabolically driven generation time of a taxon also relates functionally to species richness, and similarly, its yearly cycles reduce richness. We test these hypotheses on marine calanoid copepods with 46,377 records of data collected by scientific cruise surveys in Mediterranean regions, across which the temperature amplitudes vary dramatically. We test both bio-energetic and phenomenological effects of temperature cycles on richness in 86 1° × 1° latitudinal and longitudinal spatial units. The models incorporated the effect of both periodic fluctuations and mean temperature explained 21.6% more variation in the data, with lower AIC, compared to models incorporated only the mean temperature. The study also gives insight into the basis of energetic-equivalence rule in MTE determining richness, which can be governed by generation time of taxon. The results of this study lead to the proposition that amplitude of yearly temperature cycles may contribute to both the longitudinal and the latitudinal differences in species richness and show how the metabolic theory can explain macro-ecological patterns arising from yearly temperature cycles.







Similar content being viewed by others
References
Allen AP, Brown JH, Gillooly JF (2002) Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297(5586):1545–1548
Allen AP, Gillooly JF, Savage VM, Brown JH (2006) Kinetic effects of temperature on rates of genetic divergence and speciation. Proc Natl Acad Sci 103(24):9130–9135
Andersen V, Devey C, Gubanova A, Picheral M, Melnikov V, Tsarin S, Prieur L (2004) Vertical distributions of zooplankton across the Almeria–Oran frontal zone (Mediterranean Sea). J Plankton Res 26(3):275–293
Benyahya L, Caissie D, St-Hilaire A, Ouarda TB, Bobée B (2007) A review of statistical water temperature models. Canadian Water Resour J 32(3):179–192
Bollens, G. R., and M. R. Landry. 2000. Biological response to iron fertilization in the eastern equatorial Pacific (Iron Ex II). II. Mesozooplankton abundance, biomass, depth distribution and grazing. Mar Ecol Progress Ser 201(43-56)
Bradford-Grieve JM (2002) Colonization of the pelagic realm by calanoid copepods. Hydrobiologia 485(1–3):223–244
Burgmer T, Hillebrand H (2011) Temperature mean and variance alter phytoplankton biomass and biodiversity in a long-term microcosm experiment. Oikos 120(6):922–933
Cardillo M, Orme CDL, Owens IPF (2005) Testing for latitudinal bias in diversification rates: an example using new world birds. Ecology 86(9):2278–2287
Clarke A, Crame JA (2003) The importance of historical processes in global patterns of diversity. In: Blackburn TM, Gaston KJ (eds) Macroecology concepts and consequences. Blackwell Scientific, Oxford, pp 130–151
Colwell RK, Lees DC (2000) The mid-domain effect: geometric constraints on the geography of species richness. Trends in ecology & evolution 15(2):70–6
Currie DJ, Mittelbach GG, Cornell HV, Kaufman DM, Kerr JT, Oberdorff T (2004) Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol Lett 7:1121–1134
Damuth J (1987) Interspecific allometry of population density in mammals and other animals: the independence of body mass and population energy-use. Biol J Linn Soc 31(3):193–246
Deibel D, Lowen B (2012) A review of the life cycles and life-history adaptations of pelagic tunicates to environmental conditions. ICES J Mar Sci 69(3):358–369
Dowle EJ, Morgan-Richards M, Trewick SA (2013) Molecular evolution and the latitudinal biodiversity gradient. Heredity 110(6):501
Fox JW (2013) The intermediate disturbance hypothesis should be abandoned. Trends Ecol Evol 28(2):86–92
Gillman LN, Keeling DJ, Ross HA, Wright SD. (2009) Latitude, elevation and the tempo of molecular evolution in mammals. Proceedings of the Royal Society of London B: Biological Sciences 276(1671):3353–9
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293(5538):2248–2251
Hawkins BA, Albuquerque FS, Araujo MB, Beck J, Bini LM, Cabrero-Sanudo FJ et al (2007) A global evaluation of metabolic theory as an explanation for terrestrial species richness gradients. Ecology 88(8):1877–1888
Hays GC, Kennedy H, Frost BW (2001) Individual variability in diel vertical migration of a marine copepod: why some individuals remain at depth when others migrate. Limnol Oceanogr:2050–2054
Huntley ME, Lopez MD (1992) Temperature-dependent production of marine copepods: a global synthesis. Am Nat:201–242
IBSS (n.d.). Institute of Biology of the Southern Seas. Retrieved from: http://www.Iobis.Org/, or http://www.Marbef.Org/data/imis.Php?Module=dataset&dasid=1675 in march 2013
Jensen JLWV (1906) Sur les fonctions convexes et les inégalités entre les valeurs moyennes. Acta Mathematica 30(1):175–193
Kadane JB, Lazar NA (2004) Methods and criteria for model selection. J Am Stat Assoc 99(465):279–290
Kiørboe T, Sabatini M (1994) Reproductive and life cycle strategies in egg-carrying cyclopoid and free-spawning calanoid copepods. J Plankton Res 16(10):1353–1366
Lande R, Engen S, Saether BE (2003) Stochastic population dynamics in ecology and conservation. Oxford University Press, Oxford, p 212
Landry MR (1975) Seasonal temperature effects and predicting development rates of marine copepod eggs. Limnol Oceanography 20(3):434–440
Louys, J., Wilkinson, D. M., and L. C. Bishop. 2012. Ecology needs a paleontological perspective. In: Paleontology in ecology and conservation. Springer Berlin Heidelberg, p. 23–38
LU (n.d.), Lebanese University Retrieved from: http://www.iobis.org/. in March 2016
Maas AE, Wishner KF, Seibel BA (2012) Metabolic suppression in thecosomatous pteropods as an effect of low temperature and hypoxia in the eastern tropical North Pacific. Mar Biol 159(9):1955–1967
Mackas DL, Sefton H, Miller CB, Raich A (1993) Vertical habitat partitioning by large calanoid copepods in the oceanic subarctic Pacific during spring. Prog Oceanogr 32(1):259–294
Mauchline J, Mauchline J (1998) The biology of calanoid copepods. Academic press U.K, p 710
Mayhew PJ, Bell MA, Benton TG, McGowan AJ (2012) Biodiversity tracks temperature over time. Proc Natl Acad Sci 109(38):15141–15145
Moran PAP (1950) A test for serial independence of residuals. Biometrika 37:178–181
Nowaczyk A, Carlotti F, Thibault-Botha D, Pagano M (2011) Distribution of epipelagic metazooplankton across the Mediterranean Sea during the summer BOUM cruise. Biogeosciences 8(8):2159
Oberdoff T, Guégan JF, Hugueny B (1995) Global scale patterns of fish species richness in rivers. Ecography 18(4):345–352
Patanasatienkul T, Revie CW, Davidson J, Sanchez J (2014) Mathematical model describing the population dynamics of Ciona intestinalis, a biofouling tunicate on mussel farms in Prince Edward Island, Canada. Manag Biol Invasions 5(1):39–54
Pianka ER (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100(910):33–46
Price CA, Weitz JS, Savage VM, Stegen J, Clarke A, Coomes DA, Dodds PS, Etienne RS, Kerkhoff AJ, McCulloh K, Niklas KJ (2012) Testing the metabolic theory of ecology. Ecology Letters 15(12):1465–1474
Rajakaruna H, Lewis M (2017) Temperature cycles affect colonization potential of calanoid copepods. J Theor Biol 419:77–89
Record NR, Pershing AJ, Maps F (2012) First principles of copepod development help explain global marine diversity patterns. Oecologia 170(2):289–295
Renema W, Bellwood DR, Braga JC, Bromfield K, Hall R, Johnson KG, Pandolfi JM (2008) Hopping hotspots: global shifts in marine biodiversity. Science 321(5889):654–657
Rohde K (1992) Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65(3):514–527
Rolland J, Condamine FL, Jiguet F, Morlon H (2014) Faster speciation and reduced extinction in the tropics contribute to the mammalian latitudinal diversity gradient. PLoS Biol 12:e1001775
Rombouts I, Beaugrand G, Ibaňez F, Chiba S, Legendre L (2011) Marine copepod diversity patterns and the metabolic theory of ecology. Oecologia 166(2):349–355
Rombouts I, Beaugrand G, Ibaňez F, Gasparini S, Chiba S, Legendre L (2009) Global latitudinal variations in marine copepod diversity and environmental factors. Proc R Soc B Biol Sci 276(1670):3053–3062
Ruel JJ, Ayres MP (1999) Jensen’s inequality predicts effects of environmental variation. Trends Ecol Evol 14(9):361–366
Savage VM (2004) Improved approximations to scaling relationships for species, populations, and ecosystems across latitudinal and elevational gradients. J Theor Biol 227(4):525–534
Savage VM, Gillooly JF, Brown JH, West GB, Charnov EL (2004) Effects of body size and temperature on population growth. Am Nat 163(3):429–441
Seibel, B. A., Schneider, J. L., Kaartvedt, S., Wishner, K. F., and K. L. Daly. 2016. Hypoxia tolerance and metabolic suppression in oxygen minimum zone Euphausiids: implications for ocean deoxygenation and biogeochemical cycles. Integ Compar Biol
Shurin JB, Winder M, Adrian R, Keller WB, Matthews B, Paterson AM, Paterson MJ, Pinel-Alloul B, Rusak JA, Yan ND (2010) Environmental stability and lake zooplankton diversity—contrasting effects of chemical and thermal variability. Ecol Lett 13(4):453–463
Sommer U (1995) An experimental test of the intermediate disturbance hypothesis using cultures of marine phytoplankton. Limnol Oceanogr 40:1271–1277
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson MAX, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57(7):573–583
Subramoniam T (2016) Sexual biology and reproduction in crustaceans. Academic Press, p 526
Svetlichny LS, Hubareva ES, Erkan F, Gucu AC (2000) Physiological and behavioral aspects of Calanus euxinus females (Copepoda: Calanoida) during vertical migration across temperature and oxygen gradients. Mar Biol 137(5–6):963–971
Terborgh J (1973) On the notion of favorableness in plant ecology. Am Nat 107(956):481–501
Thomas JA, Welch JJ, Lanfear R, Bromham L (2010) A generation time effect on the rate of molecular evolution in invertebrates. Molecular biology and evolution 27(5):1173–80
Townsend CR, Scarsbrook MR, Dolédec S (1997) The intermediate disturbance hypothesis, refugia, and biodiversity in streams. Limnol Oceanogr 42(5):938–949
Weir JT, Schluter D (2007) The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315(5818):1574–1576
Willig MR, Kaufman DM, Stevens RD (2003) Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Ann Rev Ecol Evol S 34:273–309
WOA (n.d.). World Ocean Atlas. NOAA. http://www.nodc.noaa.gov/OC5/SELECT/ woaselect/ woaselect. html
Wright S, Keeling J, Gillman L (2006) The road from Santa Rosalia: a faster tempo of evolution in tropical climates. Proceedings of the National Academy of Sciences 103:7718–7722
Wright SD, Gillman LN, Ross HA, Keeling DJ (2010) Energy and the tempo of evolution in amphibians. Global Ecology and Biogeography 19:733–740
Acknowledgements
The authors thank the anonymous reviewers of the manuscript greatly. HR gratefully acknowledges the research support provided by the Mathematical Biology Unit at Okinawa Institute of Science and Technology Graduate University (OIST) of Japan. ML thanks NSERC Discovery and Accelerator grants (ML) a Killam Research Fellowship (ML) and a Canada Research Chair. The authors also thank Dr. Steven Aird at OIST for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: Calibrating generation timesg T
Appendix 1: Calibrating generation timesg T
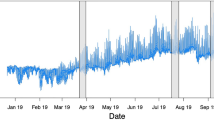
We calibrated the mean calanoid generation time per species, g T , at temperature T (°C) using generation time data of copepods from Huntley and Lopez (1992) simplifying the model Eq. (8), \( {g}_T={g}_0\overline{M^{1/4}}{\mathrm{exp}}^{E/ kT} \), as g T = ℏ 1 exp[(ℏ 2/1000)(1000/T)], with the selection of parameters, \( {\hslash}_1={g}_0\overline{M^{1/4}} \), ℏ 2 = E/k, and using the nonlinear least squares regression method lsqcurvefit in Matlab. The generation time data are given for 181 estimates in Huntley and Lopez (1992) of 33 marine copepod species tested at environmental temperatures ranging from − 1.7 to 30.7 °C. In our model calibrations, we should get ℏ 2/1000 ~ 9K, ideally, as the activation energy E for aquatic taxa is ~ 0.78 eV (Allen et al. 2002). For our study of calanoid, we assumed that g T calibrated based on marine copepod data, yielding R 2 = 0.88 (Fig. 5a), are not deviated largely from that of calanoid copepods, which usually comprises of 55–95% of the plankton samples (see Mauchline and Mauchline 1998). We assumed also that size variation among calanoid species is negligible (most are 0.5–2.0 mm in length: Mauchline and Mauchline [1998]), and the distribution is Gaussian. As it is said that the generation time is a reasonable period for acclimatization of copepods in temperature-related experiments (Landry 1975; Huntley and Lopez 1992), we assumed that metabolism, and, thus, \( {\overline{g}}_T \), responds to temperatures averaged at the generation time or a larger time-scale. As the generation time of marine copepods in the sampling units of the Mediterranean and adjacent seas, computed using the calibrated model as above, on the basis of the mean temperatures of the units, was less than 30 days (Fig. 5b), the assumption that monthly mean temperatures of the habitats is a reasonable scale, at which the copepods respond metabolically to varying temperature, may be justified.
Rights and permissions
About this article
Cite this article
Rajakaruna, H., Lewis, M. Do yearly temperature cycles reduce species richness? Insights from calanoid copepods. Theor Ecol 11, 39–53 (2018). https://doi.org/10.1007/s12080-017-0347-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-017-0347-y