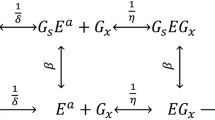Abstract
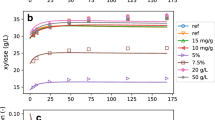
The objective of this study is to perform a comprehensive enzyme kinetics analysis in view of validating and consolidating a semimechanistic kinetic model consisting of homogeneous and heterogeneous reactions for enzymatic hydrolysis of lignocellulosic biomass proposed by the U.S. National Renewable Energy Laboratory (Kadam et al., Biotechnol Prog 20(3):698–705, 2004) and its variations proposed in this work. A number of dedicated experiments were carried out under a range of initial conditions (Avicel® versus pretreated barley straw as substrate, different enzyme loadings and different product inhibitors such as glucose, cellobiose and xylose) to test the hydrolysis and product inhibition mechanisms of the model. A nonlinear least squares method was used to identify the model and estimate kinetic parameters based on the experimental data. The suitable mathematical model for industrial application was selected among the proposed models based on statistical information (weighted sum of square errors). The analysis showed that transglycosylation plays a key role at high glucose levels. It also showed that the values of parameters depend on the selected experimental data used for parameter estimation. Therefore, the parameter values are not universal and should be used with caution. The model proposed by Kadam et al. (Biotechnol Prog 20(3):698–705, 2004) failed to predict the hydrolysis phenomena at high glucose levels, but when combined with transglycosylation reaction(s), the prediction of cellulose hydrolysis behaviour over a broad range of substrate concentrations (50–150 g/L) and enzyme loadings (15.8–31.6 and 1–5.9 mg protein/g cellulose for Celluclast and Novozyme 188, respectively) was possible. This is the first study introducing transglycosylation into the semimechanistic model. As long as these type of models are used within the boundary of their validity (substrate type, enzyme source and substrate concentration), they can support process design and technology improvement efforts at pilot and full-scale studies.










Similar content being viewed by others
Abbreviations
- BG:
-
β-Glucosidase
- CBH:
-
Exo-1,4-β-d-glucanases
- Cel:
-
Celluclast 1.5 L
- DP:
-
Degree of polymerization
- EG:
-
Endo-1,4-β-D-glucanase
- E iT :
-
Total enzyme concentration (gram protein per liter) (i = 1 for Cel; i = 2 for N188)
- E iB :
-
Bound enzyme concentration (i = 1 for Cel; i = 2 for N188)
- E iF :
-
Concentration of free enzyme in solution (i = 1 for Cel; i = 2 for N188)
- E imax :
-
Maximum mass of enzyme that adsorbs onto a unit mass of substrate (gram protein per gram substrate)
- G i :
-
Glucose (i = 1) cellobiose (i = 2), trisaccharide (i = 3) and tetrasaccharide (i = 4) concentration (grams per liter)
- G cr,tri :
-
Critical glucose concentration of transglycosylation for trisaccharide production (grams per liter)
- G cr,tetra :
-
Critical glucose concentration of transglycosylation for tetrasaccharide production (grams per liter)
- K iad :
-
Dissociation constant for enzyme adsorption/desorption reaction (liters per gram protein) (i = 1 for Cel; i = 2 for N188)
- K 3M :
-
Substrate (cellobiose) saturation constants (grams per liter)
- K iIG2 :
-
Inhibition constant cellobiose (grams per liter) (i = 1 for r 1; i = 2 for r 2)
- K iIG :
-
Inhibition constant glucose (grams per liter) (i = 1 for r 1; i = 2 for r 2; i = 3 for r 3)
- K iIX :
-
Inhibition constant xylose (grams per liter) (i = 1 for r 1; i = 2 for r 2; i = 3 for r 3)
- k ir :
-
Reaction rate constant (i = 1 and 2 liters per gram per hour; i = 3 per hour)
- k G3 :
-
Reaction rate constant of transglycosylation for trisaccharide production
- k G4 :
-
Reaction rate constant of transglycosylation for tetrasaccharide production
- N188:
-
Novozym 188
- r i :
-
Reaction rate (grams per liter per hour) (i = 1 for cellulose to cellobiose; i = 2 for cellulose to glucose; i = 3 for cellobiose to glucose)
- r tri :
-
Overall reaction rate (grams per liter per hour) of 3G ↔ G 3 + 2H2O
- r tri+ :
-
Reaction rate (grams per liter per hour) of 3G → G 3 + 2H2O
- r tri− :
-
Reaction rate (grams per liter per hour) of 3G ← G 3 + 2H2O
- r tetra :
-
Overall reaction rate (grams per liter per hour) of G + G 3 ↔ G 4 + H2O
- r tetra+ :
-
Reaction rate (grams per liter per hour) of G + G 3 → G 4 + H2O
- r tetra− :
-
Reaction rate (grams per liter per hour) of G + G 3 ← G 4 + H2O
- R S :
-
Substrate reactivity
- S :
-
Substrate concentration (grams per liter) (suffix with “0” means initial substrate concentration)
- X :
-
Xylose concentration (grams per liter)
- Xbg:
-
A BG other than N188
- α :
-
Dimensionless constant for substrate reactivity
References
Gnansounou, E. (2010). Production and use of lignocellulosic bioethanol in Europe: current situation and perspectives. Bioresource Technology, 101(13), 4842–4850.
Larsen, J., Haven, Ø. M., & Thirup, L. (2012). Inbicon makes lignocellulosic ethanol a commercial reality. Biomass and Bioenergy, 46, 36–45.
Quinlan, R. J., Sweeney, M. D., Lo Leggio, L., Otten, H., Poulsen, J. C. N., Johansen, K. S., et al. (2011). Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. PNAS, 108, 15079–15084.
Zhang, Y.-H. P., & Lynd, L. R. (2004). Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnology and Bioengineering, 88(7), 797–824.
Gan, Q., Allen, S. J., & Taylor, G. (2003). Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: an overview, an experimental study and mathematical modelling. Process Biochemistry, 38(7), 1003–1018.
Gharpuray, M. M., Lee, Y.-H., & Fan, L. T. (1983). Structural modification of lignocellulosics by pretreatments to enhance enzymatic hydrolysis. Biotechnology and Bioengineering, 25(1), 157–172.
Kadam, K. L., Rydholm, E. C., & McMillan, J. D. (2004). Development and validation of a kinetic model for enzymatic saccharification of lignocellulosic biomass. Biotechnology Progress, 20(3), 698–705.
Movagarnejad, K., Sohrabi, M., Kaghazchi, T., & Vahabzadeh, F. (2000). A model for the rate of enzymatic hydrolysis of cellulose in heterogeneous solid-liquid systems. Biochemical Engineering Journal, 4(3), 197–206.
Philippidis, G. P., Smith, T. K., & Wyman, C. E. (1993). Study of the enzymatic hydrolysis of cellulose for production of fuel ethanol by the simultaneous saccharification and fermentation process. Biotechnology and Bioengineering, 41(9), 846–853.
Sin, G., Meyer, A. S., & Gernaey, K. V. (2010). Assessing reliability of cellulose hydrolysis models to support biofuel process design—identifiability and uncertainty analysis. Computers & Chemical Engineering, 34(9), 1385–1392.
Yang, B., Willies, D. M., & Wyman, C. E. (2006). Changes in the enzymatic hydrolysis rate of Avicel cellulose with conversion. Biotechnology and Bioengineering, 94(6), 1122–1128.
Zheng, Y., Pan, Z., Zhang, R., & Jenkins, B. M. (2009). Kinetic modeling for enzymatic hydrolysis of pretreated creeping wild ryegrass. Biotechnology and Bioengineering, 102(6), 1558–1569.
Andrić, P., Meyer, A. S., Jensen, P. A., & Dam-Johansen, K. (2010). Effect and modeling of glucose inhibition and in situ glucose removal during enzymatic hydrolysis of pretreated wheat straw. Applied Biochemistry and Biotechnology, 160(1), 280–297.
Andrić, P., Meyer, A. S., Jensen, P. A., & Dam-Johansen, K. (2010). Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnology Advances, 28, 308–324.
Bhiri, F., Chaabouni, S., Limam, F., Ghrir, R., & Marzouki, N. (2008). Purification and biochemical characterization of extracellular β-glucosidases from the hypercellulolytic Pol6 mutant of Penicillium occitanis. Applied Biochemistry and Biotechnology, 149(2), 169–182.
Gusakov, A. V., Sinitsyn, A. P., Goldsteins, G. H., & Klyosov, A. A. (1984). Kinetics and mathematical model of hydrolysis and transglycosylation catalysed by cellobiase. Enzyme and Microbial Technology, 6(6), 275–282.
Kono, H., Waelchli, M. R., Fujiwara, M., Erata, T., & Takai, M. (1999). Transglycosylation of cellobiose by partially purified Trichoderma viride cellulase. Carbohydrate Research, 319(1–4), 29–37.
Pal, S., Banik, S. P., Ghorai, S., Chowdhury, S., & Khowala, S. (2010). Purification and characterization of a thermostable intra-cellular β-glucosidase with transglycosylation properties from filamentous fungus Termitomyces clypeatus. Bioresource Technology, 101(7), 2412–2420.
Watanabe, T., Sato, T., Yoshioka, S., Koshijima, T., & Kuwahara, M. (1992). Purification and properties of Aspergillus niger β-glucosidase. European Journal of Biochemistry, 209(2), 651–659.
Morales-Rodriguez, R., Meyer, A. S., Gernaey, K. V., & Sin, G. (2012). A framework for model-based optimization of bioprocesses under uncertainty: lignocellulosic ethanol production case. Computers & Chemical Engineering, 42, 115–129.
Rosgaard, L., Pedersen, S., & Meyer, A. S. (2007). Comparison of different pretreatment strategies for enzymatic hydrolysis of wheat and barley straw. Applied Biochemistry and Biotechnology, 143(3), 284–296.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2008). Determination of structural carbohydrate and lignin in biomass. Laboratory analytical procedure. NREL/TP-510–42618. Updated 2010.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257–268.
Sternberg, D., Vijayakumar, P., & Reese, E. T. (1977). β-Glucosidase: microbial production and effect on enzymatic hydrolysis of cellulose. Canadian Journal of Microbiology, 23(2), 139–147.
Drissen, R. E. T., Maas, R. H. W., Van Dermaarel, M. J. E. C., Kabel, M. K., Schols, H. A., Tramper, J., et al. (2007). A generic model for glucose production from various cellulose sources by a commercial cellulase complex. Biocatalysis and Biotransformation, 25(6), 419–429.
Ooshima, H., Kurakake, M., Kato, J., & Harano, Y. (1991). Enzymatic activity of cellulase adsorbed on cellulose and its change during hydrolysis. Applied Biochemistry and Biotechnology, 31(3), 253–266.
Medve, J., Karlsson, J., Lee, D., & Tjerneld, F. (1998). Hydrolysis of microcrystalline cellulose by cellobiohydrolase I and endoglucanase II from Trichoderma reesei: adsorption, sugar production pattern, and synergism of the enzymes. Biotechnology and Bioengineering, 59(5), 621–634.
Nidetzky, B., Zachariae, W., Gercken, G., Hayn, M., & Steiner, W. (1994). Hydrolysis of cellooligosaccharides by Trichoderma reesei cellobiohydrolases: experimental data and kinetic modeling. Enzyme and Microbial Technology, 16(1), 43–52.
Morales-Rodriguez, R., Meyer, A. S., Gernaey, K. V., & Sin, G. (2011). Dynamic model-based evaluation of process configurations for integrated operation of hydrolysis and co-fermentation for bioethanol production from lignocellulose. Bioresource Technology, 102(2), 1174–1184.
Acknowledgments
R. Morales-Rodriguez acknowledges the Mexican National Council for Science and Technology (CONACyT, project #145066) for the financial support for the development of part of this project. We acknowledge the Novozymes BioProcess Academy at The Technical University of Denmark for financial support and G. Balduck for contributing to some of the experimental work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1169 kb)
Rights and permissions
About this article
Cite this article
Tsai, CT., Morales-Rodriguez, R., Sin, G. et al. A Dynamic Model for Cellulosic Biomass Hydrolysis: a Comprehensive Analysis and Validation of Hydrolysis and Product Inhibition Mechanisms. Appl Biochem Biotechnol 172, 2815–2837 (2014). https://doi.org/10.1007/s12010-013-0717-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0717-x