Abstract
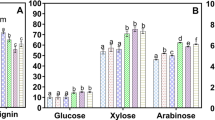
Feedstock particle sizing can impact the economics of cellulosic ethanol commercialization through its effects on conversion yield and energy cost. Past studies demonstrated that particle size influences biomass enzyme digestibility to a limited extent. Physical size reduction was able to increase conversion rates to maximum of ≈50%, whereas chemical modification achieved conversions of >70% regardless of biomass particle size. This suggests that (1) mechanical pretreatment by itself is insufficient to attain economically feasible biomass conversion, and, therefore, (2) necessary particle sizing needs to be determined in the context of thermochemical pretreatment employed for lignocellulose conversion. Studies of thermochemical pretreatments that have taken into account particle size as a factor have exhibited a wide range of maximal sizes (i.e., particle sizes below which no increase in pretreatment effectiveness, measured in terms of the enzymatic conversion resulting from the pretreatment, were observed) from <0.15 to 50 mm. Maximal sizes as defined above were dependent on the pretreatment employed, with maximal size range decreasing as follows: steam explosion > liquid hot water > dilute acid and base pretreatments. Maximal sizes also appeared dependent on feedstock, with herbaceous or grassy biomass exhibiting lower maximal size range (<3 mm) than woody biomass (>3 mm). Such trends, considered alongside the intensive energy requirement of size reduction processes, warrant a more systematic study of particle size effects across different pretreatment technologies and feedstock, as a requisite for optimizing the feedstock supply system.


Similar content being viewed by others
References
Perlack, R. D., Wright, L. L., Turhollow, A. F., Graham, R. L., Stokes, B. J., & Erbach, D. C. (2005). Biomass as feedstock for a bioenergy and bioproducts industry: the technical feasibility of a billion-ton annual supply. Oak Ridge: Oak Ridge National Laboratory.
Vertès, A. A., Inui, M., & Yukawa, H. (2008). Technological options for biological fuel ethanol. Journal of Molecular Microbiology and Biotechnology, 15, 16–30.
Meunier-Goddik, L., Bothwell, M., Sangseethong, K., Piyachomkwan, K., Chung, Y.-C., Thammasouk, K., et al. (1999). Physicochemical properties of pretreated poplar feedstock during simultaneous saccharification and fermentation. Enzyme Microbial Technol, 24, 667–674.
Mooney, C. A., Mansfield, S. D., Touhy, M. G., & Saddler, J. N. (1998). The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresource Technology, 64, 113–119.
Foust, T. D., Ibsen, K. N., Dayton, D. C., Hess, J. R., & Kenney, K. E. (2008). The biorefinery. In M. E. Himmel (Ed.), Biomass recalcitrance: deconstructing the plant cell wall for bioenergy (pp. 7–37). Oxford: Blackwell.
Zwart, R. W. R., Boerrigter, H., & van der Drift, A. (2006). The impact of biomass pretreatment on the feasibility of overseas biomass conversion to Fischer–Tropsch products. Energ Fuel, 20, 2192–2197.
Mansfield, S. D., Mooney, C., & Saddler, J. N. (1999). Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnology Progress, 15, 804–816.
Zhang, Y.-H. P., & Lynd, L. R. (2004). Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnology and Bioengineering, 88, 797–824.
Ding, S.-Y., & Himmel, M. E. (2008). Anatomy and ultrastructure of maize cell walls: an example of energy plants. In M. E. Himmel (Ed.), Biomass recalcitrance: deconstructing the plant cell wall for bioenergy (pp. 38–60). Oxford: Blackwell.
Harris, P. J., & Stone, B. A. (2008). Chemistry and molecular organization of plant cell walls. In M. E. Himmel (Ed.), Biomass recalcitrance: deconstructing the plant cell wall for bioenergy (pp. 61–93). Oxford: Blackwell.
Chang, V. S., & Holtzapple, M. T. (2000). Fundamental factors affecting biomass enzymatic reactivity. Applied Biochemistry and Biotechnology, 84–86, 5–37.
Sinitsyn, A. P., Gusakov, A. V., & Vlasenko, E. Y. (1991). Effect of structural and physico-chemical features of cellulosic substrates on the efficiency of enzymatic hydrolysis. Applied Biochemistry and Biotechnology, 30, 43–59.
Grethlein, H. E. (1985). The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates. Biotechnol, 3, 155–160.
Thompson, D. N., Chen, H. C., & Grethlein, H. E. (1992). Comparison of pretreatment methods on the basis of available surface area. Bioresource Technology, 39, 155–163.
Gharpuray, M. M., Lee, Y.-H., & Fan, L. T. (1983). Structural modifications of lignocellulosics by pretreatment to enhance enzymatic hydrolysis. Biotechnology and Bioengineering, 25, 157–172.
Himmel, M., Tucker, M., Baker, J., Rivard, C., Oh, K., & Grohmann, K. (1985). Comminution of biomass: hammer and knife mills. Biotechnology and Bioengineering Symposium, 15, 39–58.
Cadoche, L., & López, G. D. (1989). Assessment of size reduction as a preliminary step in the production of ethanol from lignocellulosic wastes. Biol Wastes, 30, 153–157.
Schell, D. J., & Harwood, C. (1994). Milling of lignocellulosic biomass: results of pilot-scale testing. Applied Biochemistry and Biotechnology, 45(46), 159–168.
Mani, S., Tabil, L. G., & Sokhansanj, S. (2004). Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass and Bioenergy, 27, 339–352.
Bitra, V. S. P., Womac, A. R., Chevanan, N., Miu, P. I., Igathinathane, C., Sokhansanj, S., et al. (2009). Direct mechanical energy measures of hammer mill comminution of switchgrass, wheat straw, and corn stover and analysis of their particle size distributions. Powder Technology, 193, 32–45.
Esteban, L. S., & Carrasco, J. E. (2006). Evaluation of different strategies for pulverization of forest biomasses. Powder Technology, 166, 139–151.
Aden, A., Ruth, M., Ibsen, K., Jechura, J., Neeves, K., Sheehan, J., et al. (2002). Lignocellulosic biomass to ethanol process design and economics utilizing co-current dilute acid prehydrolysis and enzymatic hydrolysis for corn stover. Golden: National Renewable Energy Laboratory.
Holtzapple, M. T., Humphrey, A. E., & Taylor, J. D. (1989). Energy requirements for the size reduction of poplar and aspen wood. Biotechnology and Bioengineering, 33, 207–210.
Zhu, J. Y., Wang, G. S., Pan, X. J., & Gleisner, R. (2009). Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chemical Engineering Science, 64, 474–485.
Shewale, I. G., & Sadana, J. C. (1979). Enzymatic hydrolysis of cellulosic materials by Sclerotium rolfsii culture filtrate for sugar production. Canadian Journal of Microbiology, 25, 773–783.
Weimer, P. J., & Weston, W. M. (1985). Relationship between the fine structure of native cellulose degradability by the cellulase complexes of Trichoderma reesei and Clostridium thermocellum. Biotechnology and Bioengineering, 27, 1540–1547.
Puri, V. P. (1984). Effect of crystallinity and degree of polymerization of cellulose on enzymatic saccharification. Biotechnology and Bioengineering, 26, 1219–1222.
Ryu, D. D. Y., Lee, S. B., Tassinari, T., & Macy, C. (1982). Effect of compression milling on cellulose structure and on enzymatic hydrolysis kinetics. Biotechnology and Bioengineering, 24, 1047–1067.
Caulfield, D. F., & Moore, W. E. (1974). Effect of varying crystallinity of cellulose on enzymatic hydrolysis. Wood Sci, 6, 375–379.
Rivers, D. B., & Emert, G. H. (1987). Lignocellulose pretreatment: a comparison of wet and dry ball attrition. Biotechnological Letters, 9, 365–368.
Rivers, D. B., & Emert, G. H. (1988). Factors affecting the enzymatic hydrolysis of municipal solid waste components. Biotechnology and Bioengineering, 26, 278–281.
Rivers, D. B., & Emert, G. H. (1988). Factors affecting the enzymatic hydrolysis of bagasse and rice straw. Biol Wastes, 26, 85–95.
Pordesimo, L. O., Ray, S. J., Buschermohle, M. J., Waller, J. C., & Wilkerson, J. B. (2005). Processing cotton gin trash to enhance in vitro dry matter digestibility in reduced time. Bioresource Technology, 96, 47–53.
Düsterhöft, E.-M., Engels, F. M., & Voragen, A. G. J. (1993). Parameters affecting the enzymic hydrolysis of oil-seed meals, lignocellulosic by-products of the food industry. Bioresource Technology, 44, 39–46.
Dasari, R. K., & Berson, R. E. (2007). The effect of particle size on hydrolysis reaction rates and rheological properties in cellulosic slurries. Applied Biochemistry and Biotechnology, 137, 289–299.
Elshafei, A. M., Vega, J. L., Klasson, K. T., Clausen, E. C., & Gaddy, J. L. (1991). The saccharification of corn stover by cellulase from Penicillium funiculosum. Bioresource Technology, 35, 73–80.
Mooney, C. A., Mansfield, S. D., Beatson, R. P., & Saddler, J. N. (1999). The effect of fiber characteristics on hydrolysis and cellulase accessibility to softwood substrates. Enzyme Microbial Technol, 25, 644–650.
Jackson, L. S., Heitmann, J., & Joyce, T. (1993). Enzymatic modifications of secondary fibre. Tappi Journal, 76, 147–154.
Laivins, G. V., & Scallan, A. M. (1996). The influence of drying and beating on the swelling of fines. Journal of Pulp and Paper Science, 22, J178–J184.
Nazhad, M. M., Ramos, L. P., Paszner, L., & Saddler, J. N. (1995). Structural constraints affecting the initial enzymatic hydrolysis of recycled paper. Enzyme and Microbial Technology, 17, 68–74.
Hendriks, A. T. W. M., & Zeeman, G. (2009). Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresource Technology, 100, 10–18.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., et al. (2005). Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresource Technology, 96, 673–686.
Eggeman, T., & Elander, R. T. (2005). Process and economic analysis of pretreatment technologies. Bioresource Technology, 96, 2019–2025.
Chheda, J. N., Román-Leshkov, Y., & Dumesic, J. A. (2007). Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chemistry, 9, 342–350.
Kim, S. B., & Lee, Y. Y. (2002). Diffusion of sulfuric acid within lignocellulosic biomass particles and its impact on dilute-acid pretreatment. Bioresource Technology, 83, 165–171.
Carrasco, F., & Roy, C. (1992). Kinetic study of dilute-acid prehydrolysis of xylan-containing biomass. Wood Science and Technology, 26, 189–208.
Maloney, M. T. (1986). An engineering analysis of the production of xylose by dilute-acid hydrolysis of hardwood hemicellulose. Biotechnology Progress, 2, 192–202.
Aguilar, R., Ramírez, J. A., Garrote, G., & Vázquez, M. (2002). Kinetic study of the acid hydrolysis of sugar cane bagasse. Journal of Food Engineering, 55, 309–318.
Bhandari, N., MacDonald, D. G., & Bakhshi, N. N. (1984). Kinetic studies of corn stover saccharification using sulphuric acid. Biotechnology and Bioengineering, 26, 320–327.
Brennan, A. H., Hoagland, W., & Schell, D. J. (1986). High temperature acid hydrolysis of biomass using an engineering-scale plug flow reactor: results of low solids testing. Biotechnology and Bioengineering Symposium, 17, 53–70.
Ranganathan, D. G., MacDonald, D. G., & Bakhshi, N. N. (1985). Kinetic studies of wheat straw hydrolysis using sulphuric acid. Canadian Journal of Chemical Engineering, 63, 840–844.
Singh, A., Das, K., & Sharma, D. K. (1984). Production of xylose, furfural, fermentable sugars and ethanol from agricultural residues. Journal of Chemical Technology and Biotechnology, 34A, 51–61.
Springer, E. L. (1985). Prehydrolysis of hardwoods with dilute sulfuric acid. Industrial & Engineering Chemistry Product Research and Development, 24, 614–623.
Yat, S. C., Berger, A., & Shonnard, D. R. (2008). Kinetic characterization for dilute sulfuric acid hydrolysis of timber varieties and switchgrass. Bioresource Technology, 99, 3855–3863.
Hsu, T.-A., Himmel, M., Schell, D., Farmer, J., & Berggren, M. (1996). Design and initial operation of a high-solids, pilot-scale reactor for dilute-acid pretreatment of lignocellulosic biomass. Applied Biochemistry and Biotechnology, 57(58), 3–18.
Mason WH. Process and apparatus for disintegration of wood and the like. US Patent 1,578,609;1926
Brownell, H. H., Yu, E. K. C., & Saddler, J. N. (1986). Steam-explosion pretreatment of wood: effect of chip size, acid, moisture content and pressure drop. Biotechnology and Bioengineering, 28, 792–801.
Gregg, D. J., & Saddler, J. N. (1996). Factors affecting cellulose hydrolysis and the potential of enzyme recycle to enhance the efficiency of an integrated wood to ethanol process. Biotechnology and Bioengineering, 51, 375–383.
Cullis, I. F., Saddler, J. N., & Mansfield, S. D. (2004). Effect of initial moisture content and chip size on the bioconversion efficiency of softwood lignocellulosics. Biotechnology and Bioengineering, 85, 413–421.
Ballesteros, I., Oliva, J. M., Navarro, A. A., Gonzalez, A., Carrasco, J., & Ballesteros, M. (2000). Effect of chip size on steam explosion pretreatment of softwood. Applied Biochemistry and Biotechnology, 84–86, 97–110.
Ballesteros, I., Oliva, J. M., Negro, M. J., Manzanares, P., & Ballesteros, M. (2002). Enzymic hydrolysis of steam exploded herbaceous agricultural waste (Brassica carinata) at different particle sizes. Process Biochemistry, 38, 187–192.
Negro, M. J., Manzanares, P., Ballesteros, I., Oliva, J. M., Cabañas, A., & Ballesteros, M. (2003). Hydrothermal pretreatment conditions to enhance ethanol production from poplar biomass. Applied Biochemistry and Biotechnology, 105–108, 87–100.
van Walsum, G. P., Allen, S. G., Spencer, M. J., Laser, M. S., Antal, M. J., Jr., & Lynd, L. R. (1996). Conversion of lignocellulosics pretreated with liquid hot water to ethanol. Applied Biochemistry and Biotechnology, 57(58), 157–170.
Laureano-Perez, L., Teymouri, F., Alizadeh, H., & Dale, B. E. (2005). Understanding factors that limit enzymatic hydrolysis of biomass: characterization of pretreated corn stover. Applied Biochemistry and Biotechnology, 121–124, 1081–1099.
Chundawat, S. P. S., Venkatesh, B., & Dale, B. E. (2007). Effect of particle size based separation of milled corn stover on AFEX pretreatment and enzymatic digestibility. Biotechnology and Bioengineering, 96, 219–231.
Moniruzzaman, M., Dale, B. E., Hespell, R. B., & Bothast, R. J. (1997). Enzymatic hydrolysis of high-moisture corn fiber pretreated by AFEX and recovery and recycling of the enzyme complex. Applied Biochemistry and Biotechnology, 67, 113–126.
Chang, V. S., Burr, B., & Holtzapple, M. T. (1997). Lime pretreatment of switchgrass. Applied Biochemistry and Biotechnology, 63(65), 3–19.
Li, Y., Ruan, R., Chen, P. L., Liu, Z., Pan, X., Lin, X., et al. (2004). Enzymatic hydrolysis of corn stover pretreated by combined dilute alkaline treatment and homogenization. T ASABE, 47, 821–825.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Critical particle sizes for dilute acid pretreatment of different biomass calculated according to the proposed model of Kim and Lee [44]. Sulfuric acid concentration = 0.5% w/w; temperature = 180°C. Critical thickness and diameter are for plate and spherical geometry, respectively. Values for k were obtained using the Arrhenius expression model for hemicellulose degradation (single hemicellulose fraction approximation) with parameters obtained from published sources:
- A o :
-
preexponential factor (c = 0)
- c :
-
acid concentration (percent w/w)
- n :
-
exponent parameter determined experimentally
- E :
-
activation energy
- R :
-
gas constant
- T :
-
temperature
Rights and permissions
About this article
Cite this article
Vidal, B.C., Dien, B.S., Ting, K.C. et al. Influence of Feedstock Particle Size on Lignocellulose Conversion—A Review. Appl Biochem Biotechnol 164, 1405–1421 (2011). https://doi.org/10.1007/s12010-011-9221-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9221-3