Abstract
Significant studies on phospholipases optimization, characterization, physiological role and industrial potential have been conducted worldwide. Some of them have been directed for biotechnological advances such as gene discovery and functional enhancement by protein engineering. Others reported phospholipases as virulence factor and major cause of pathophysiological effects. A general overview on phospholipase is needed for the identification of new reliable and efficient phospholipase, which would be potentially used in number of industrial and medical applications. Phospholipases catalyse the hydrolysis of one or more ester and phosphodiester bonds of glycerophospholipids. They vary in site of action on phospholipid which can be used industrially for modification/production of new phospholipids. Catalytically active phospholipase mainly use phosphatidylcholine as major substrate, but they can also show specificity with other phospholipids. Several accurate phospholipase assay methods are known, but a rapid and reliable method for high-throughput screening is still a challenge for efficient supply of superior phospholipases and their practical applications. Major application of phospholipase is in industries like oil refinery, health food manufacturing, dairy, cosmetics etc. All types of phospholipases can be involved as virulence factor. They can also be used as diagnostic markers for microbial infection. The importance of phospholipase in virulence is proven and inhibitors of the enzyme can be used as candidate for preventing the associated disease.
Similar content being viewed by others
Introduction
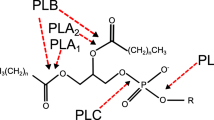
Heterogeneous groups of enzymes ‘phospholipase’ have ability to hydrolyze one or more ester linkages in glycerophospholipids. It represents a class of lipolytic enzymes, acyl hydrolyses and phosphodiesterases [1]. Although all phospholipases target phospholipids as substrates, they vary in the site of action on the phospholipids molecule, their function, mode of action and their regulation. Depending on the site of action, phospholipases are classified and qualifying letters such as A, B, C and D are used to differentiate among them and indicate the specific bond targeted in the phospholipids molecule (Table 1; Fig. 1). For example, phospholipase A1 (PLA1) hydrolyses the fatty acyl ester bond at the sn-1 position of the glycerol moiety. A complete cleavage can be achieved by all classes of phospholipases. The PLA1 and PLA2 enzyme actions produce free fatty acids and 2-acyl lysophospholipid or 1-acyl lysophospholipid, respectively. The fatty acid linked to the lysophospholipid is cleaved by lysophospholipase activity of phospholipase B (PLB). Phospholipases C (PLC) are defined as phosphodiesterases that cleave the glycerophosphate bond and finally the base group of phospholipid removed by phospholipase D [2–4]. Catalytically active phospholipases share a common substrate—phospholipids and phosphatidylcholine (PC) as major substrate. However, they can also act on phosphatidylethanolamine (PE), phosphatidylinositol (PI), sphingomyelin, lysophosphatidylcholine (LPC) and lysophosphatidylinositol (LPI) in some organisms and tissues [5]. Phospholipases are used as scientific research tool for hydrolysis, synthesis and trans-phosphatidylation, which provides various kinds of phospholipids [6]. These phospholipids have wide industrial applications in food technology, cosmetics and pharmaceuticals. Phospholipases used for industrial processes in food industry are mainly produced from porcine or bovine pancreas. Microbially produced phospholipases are used for degumming of edible oils, synthesis of chemicals and production of heath food, dairy and agricultural products (Table 2) [7, 8].
The enzyme phospholipase has considerable physiological significance. In eukaryotes, phospholipases are involved in some stages of lipid metabolism like fat digestion, reconstitution, lipoprotein metabolism etc. The phospholipases also initiate the signal transduction cascade in response to cell surface receptor activation and function as lipid second messengers [5, 9, 10]. In microbes, they are commonly believed to have a role in virulence factor. Destruction of phospholipids by pathogenic bacteria/fungal phospholipases and subsequent change of membrane constituents lead to cell damage which is regarded to be a major virulence mechanism in infection [4]. Different classes of PLs are reported from bacteria and fungi and studied for its significance in virulence and pathogenesis (Table 3) [2, 4, 9, 11–104].
Phospholipases has been the subject of some reviews that give details of specific physiological role, pathological potential, biotechnological advances and industrial significance. For example, review articles have been published on biological and pathological functions of secretory phospholipase A2 and their receptor [10], specific class of phospholipases and their role as virulence factor [4, 105, 106], their industrial applications [7], phospholipase-catalysed reactions for the production of phospholipids with industrial relevance [107, 108], occurrence, gene discovery and biotechnology [8]. The demand of new reliable and efficient new phospholipase has steadily increased over the past several decades. In this review, we will give a general overview on phospholipase classification, screening and assay methods, example of sources of different classes of phospholipases, their substrate specificity and key role to the industrial process, and finally, we will draw attention to bacterial and fungal phospholipases for pathogenicity. This will make possible the valuable means to rapidly develop their industrial and medical applications.
Phospholipase A
PLA comprises a diverse family of lipolytic enzyme produced by bacteria as well as eukaryotic cells, used to catalyse the removal of a fatty acid from phospholipids to generate lysophospholipids [104]. PLA1 hydrolyzes the sn-1 fatty acid ester bond of glycerophospholipids and PLA2 hydrolysis at the sn-2 fatty acid ester bond and reactions are shown in Fig. 2a, b. The enzyme subgroups can remove and replace the acyl chain in various phospholipids via hydrolysis, esterification and trans-esterification.
PLA1 activities have been detected in many cells and tissues from mammals, venoms and microorganisms. However, a limited number of PLA1 were purified and cloned so far [109]. PLA1 enzymes are present in both the outer membrane and cytoplasmic stage. Outer membrane PLA (OMPLA; EC 3.1.1.32) has broad substrate specificity, hydrolyzes all phospholipids, neutral glycerides and cytoplasmic PLA1, with a high degree of specificity for phosphatidylglycerol. Cytoplasmic PLA1 can be inactivated by heat and detergent and can also act as a transacylase. OMPLA is one of the few enzymes present in the outer membrane of gram-negative bacteria [105]. OMPLA is not required for the bacterial cell under laboratory conditions, but it might be essential for growth in the natural environment. This enzyme is unusually resistant to inactivation by heat and ionic detergent and requires calcium for maximal activity. Another selective function of OMPLA is related to organic solvent tolerance in the bacterium; however, the role of OMPLA in this process needs to be clarified [110].
Recently, it has been reported that PLA1 plays important biological role in both phospholipidosis—a pathological condition in which phospholipids accumulate in lysosomes and virulence factors for bacterial and fungal pathogenesis [58]. In addition to its physiological roles, PLA1 is of particular interest for industrial applications as it yields 2-acyl-lysophospholipids. Lysophospholipids are excellent emulsifiers and are particularly suitable for use in many industrial applications, such as food technology, cosmetics and pharmaceutical industries. Lysophospholipids are able to enhance emulsification in oil–water emulsions due to increased solubility in water, form emulsions which are more stable to changing pH and temperature conditions and keep emulsions stable in the presence of magnesium or calcium ions. Lysophospholipids also have several physiological functions, including a role in platelet aggregation and a role as a signalling molecule [111]. They also affect ripening and storage characteristics of fruits, leaves and green plant tissue [112]. Immobilized PLA1 was used for preparation of 1-ricinoleoyl-2-acyl-sn-glycero-3-phosphocholine from soya to egg phosphatidylcholine [7, 8]. The ricinoleic acid was incorporated at sn-1 position of egg and soybean lecithin. PLA1 is not yet widely available and provides the scope in search for superior PLA1 sources.
PLA2 has received extensive characterization and is more frequently reported enzyme in bacteria, protozoa, invertebrates and mammals (Table 3). PLA2 participates in a wide variety of physiological processes, including phospholipids digestion, signal transduction, remodelling of cell membrane and host defence. It also takes part in pathophysiological processes by producing precursors of various types of biologically active lipid mediators, such as prostaglandins, leukotrienes, thromboxanes and platelet-activating factor (PAF) [10]. Advances in molecular biology, numerous eukaryotic PLA2s have been identified and characterized. According to their biochemical features, such as cellular localization, requirement of Ca2+, substrate specificity and the primary structure, these PLA2s are classified in to three main categories, including (a) low MW (13–18 kDa) secretary PLA2, (b) Ca2+-sensitive arachidonoyl-specific (∼85 kDa) cytosolic PLA2, (c) Ca2+-independent PLA2 (85–88 kDa) and (d) several subdivision, e.g. PLA2-IB, PLA2-IIA, PAF-acetylhydrolase etc. [113]. Secreted and membrane-bound PLA2 activity has been described in bacteria, fungi and protozoa [4, 114]. This activity is reported to be calcium-dependent and related to pathogenesis in most microorganisms. The crystal structure and the tertiary structure of the secreted PLA2 (EC 3.1.1.4) from Streptomyces violaceoruber A-2688 have been determined by nuclear magnetic resonance (NMR) and X-ray analyses. Interestingly, the X-ray diffraction data of this PLA2 indicated that although the tertiary structure of the microbial PLA2 is strikingly different from that of the bovine pancreatic phospholipase, the internal motion which is associated with the calcium binding, phospholipids binding and allosteric interfacial activation is common to both [114].
Other major functions of PLA2 in the toxicity of venomous organisms [97], the virulence factor in bacterial infections [40, 105], biocatalytic uses for the phospholipid modification [6, 8] and industrially used as degumming process for refining vegetable oils [7, 115] were also discussed. Therefore, a screening program for new microbial PLA1 or PLA2 would be always required to develop as more suitable for commercial production.
Phospholipase B
Phospholipase B enzymes catalyse three distinct activities: a sn-1 and sn-2 fatty acid ester hydrolase, a lysophospholipase and a transacylase activity (Fig. 3a). Thus, it created confusion in nomenclature [84]; therefore, synonyms lysophospholipase and lysophospholipase–transacylase are used for PLB. The hydrolase activity allows the enzyme to cleave fatty acids from both phospholipids (PLB activity) and lysophospholipid (lysophospholipase activity; Fig. 3b), while the transacylase activity allows the enzyme to produce phospholipids by transferring a fatty acid to a lysophospholipid (Fig. 3a). Many fungal species appear to produce PLB with both hydrolyzing and acyltransferase activity [4, 59, 116]. These activities have also been described in bacteria [2], Dictyostelium discoideum (protozoa) [84] and in mammalian cells [87, 94, 96]. The fungal PLBs range 600–700 amino acids in length and have a potential role in virulence and in fungal pathogenesis [4]. PLB has also been cloned from mammalian cells but is not sequenced related to the fungal PLBs or bacterial PLB. The focus on potential role of PLB in pathogenesis of fungal infections is reviewed later.
Phospholipase C
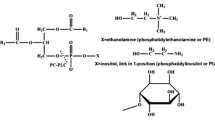
C-type phospholipases catalyse the cleavage of membrane phospholipids to 1,2-di-acyl glycerol and the respective organic phosphate (Fig. 4). According to their substrate specificities, PLC can be classified as (a) PI-specific PLC (PI-PLC), (b) PC-preferring PLC (PC-PLC) and (c) non-specific PLC. PI-PLC enzyme in eukaryotes play key role in generating membrane-associated second messengers [117, 118], and in prokaryotes, they act as virulence factor in some pathogenic bacteria [13, 30, 31]. Bacterial PI-PLCs consist of a single domain of 30–35 kDa, while the much larger eukaryotic enzymes (80–150 kDa) are organized in several distinct domains. Both the eukaryotic and prokaryotic enzymes need cofactors calcium and zinc, respectively, for optimum catalytic activity [118]. PI-PLC [117] catalyses the hydrolysis of PI into two steps: (a) intra-molecular phosphotransferase reaction to inositol 1,2-cyclic phosphate (CIP) followed by (b) a cyclic phosphodiesterase activity that converts CIP to inositol-1-phosphate. Feng et al. [119] studied the PI-PLC from Bacillus thuringiensis which exhibits several types of kinetic interfacial activation by interfaces. Micellar PI is a better substrate than monomeric PI, and interface of PC is a non-substrate (does not bind at the active site) that activates the enzyme for both PI cleavage and CIP hydrolysis. PI-PLC has seven tryptophan residues at active site domain. Two of the seven tryptophan (Try-47 and Try 242) clearly have role in PC activation of PI-PLC hydrolysis of water soluble CIP because they also affect PI cleavage to CIP. They are important for orienting the enzyme at any interface, either activating interfaces such as PC or a substrate interface (PI). Removal of both rim tryptophan produces an enzyme that is less sensitive to both activating and a substrate interfaces. PC-PLC have relatively broad specificity towards the head group of phospholipid. They have been detected in a variety of bacteria: Listeria monocytogenes [15–19] showed PLC as virulence factor while the virulence of Bacillus cereus [27–29] has not been investigated in animals and non-specific PLC of this bacterium was considered to be nontoxic (Table 3).
PLC has been mainly studied for its significance in biochemistry to the eukaryotic cell metabolism [5]. Extracellular microbial production of PLC is still at relatively low level; thus, their industrial applications are limited [7].
Phospholipase D
Phospholipase D (PLD) activities are present in prokaryotic and eukaryotic organisms including mammalian tissues and cell lines. They are involved in phospholipids metabolism, nucleases, toxins and virus envelop protein of unknown function [120, 121]. Different PLDs can catalyse the hydrolysis of PC, PE, PI, phosphatidylserine (PS), lysophophatidylcholine, sphingomyelin, phosphatidylglycerol (PG) or N-acyl phosphatidylethanolamine (NAPE), generating phosphoric acid (PA) and the accompanying polar head group (Fig. 5a). PLs also exhibit a unique trans-phosphatidylation activity that transfers the phosphatidyl moiety of the substrate of certain nucleophiles such as ethanol, thereby forming phosphatidylethanol [3, 120, 122, 123]. Thus, in trans-phosphatidyl transfer reaction, the primary alcohol acts as nucleophilic acceptor in place of water (Fig. 5b). The significant amount of PLD has been secreted by Streptomyces antibioticus [48, 124], Streptomyces septatus [125], Streptomyces halstedii [44, 45] and Streptomyces chromofuscus [46]. Their trans-phosphatidylation activity can be enzymatically used in modification of phospholipids and synthesis of phospholipid conjugates for the use of food, cosmetics and pharmaceutical industries. Although many Streptomyces strains have been isolated as high producer of extracellular PLD, the productivity has not been high enough to permit industrial application. Therefore, many researchers are still trying to isolate PLD hyper producing strains. PLD-catalysed trans-phosphatidylation reactions were performed by commercially available PLD of plant particularly cabbage and microbes mainly Streptomyces species [124], Saccharomyces cerevisiae (encoded by SPO14). PLD was described as essential for meiosis [3]. The role of PLD as virulence factor for Corynebacterium pseudotuberculosis, Corynebacterium ulcerans and Arcanobacterium haemolyticum has been reported [36, 37] as a result of targeted mutagenesis of the phospholipase D gene with decrease in virulence of C. pseudotuberculosis. Mammalian PLD has one of the key enzymes in intracellular signalling, where phosphatidic acid is greatly involved in signal transduction. Interaction of extracellular signal molecules with cell surface receptors often activates a PLD which is believed to play an important role in the regulation of cell function and cell fate.
Multiple PLD activities were characterized in eukaryotic cells and several PLD genes have been cloned [2]. Mammalian cell contain two PLD genes, PLD1 and PLD2, both of which have been cloned and overexpressed in variety of cell lines. PLD1 was found primarily on intracellular membranes (e.g. Golgi vesicles, endoplasmic reticulum, nuclear membrane etc.) and became active only when stimulated with variety of activators. It was active in vitro but inactive in vivo. PLD2 was associated mainly with plasma membrane and modestly stimulated by the known PLD activators. It showed low activity in vivo but high activity in vitro.
Signal-activated phospholipases and lipid kinases together represent the molecular basis for the rapidly growing field of lipid signalling. The activity of PLD was stimulated upon receptor ligation by agonists resulting in modification of various lipid constituents of the membrane. This was done either by degradation or by phosphorylation and generation of one or more products (messengers) through recruit or modulates specific target proteins. PC-specific PLD generated PA was considered as the major messenger molecule and choline does not have a signalling function, but it serves as a substrate for acetylcholine synthesis in neurons. Physiological function and molecular biological approaches clears its biological role by several mechanisms [120, 121]. The PA can then be dephosphorylated by PA phosphohydrolase to produce diglycerides (DG) [121]. The DG can activate protein kinase C to regulate a host of cellular responses [126] such as induction of Ca2+ influx and inhibition of adenylate cyclase in signal transduction (central component in signal transduction). PLD was also thought to be an integral part of the signalling network involving various phospholipases [120]. The various physiological processes linked to lipid second messengers (PA and DG) are secretion, vesicle trafficking mitosis and meiosis [3]. In phagocytic leukocytes (monocytes, macrophages and neutrophils), activation of PLD regulates cytoskeletal-dependent anti-microbial responses, generation of reactive oxidants and granule secretion [127]. Liscovitch et al. [120] tabulated PLD from different cell types, which were activated by various stimuli, and the main PLD activators were various hormones (e.g. vasopressin, gonadotrophin-releasing hormone), neurotransmitters (cholinergic agonists, histamine and nor adrenaline), growth factor (platelet-derived growth factor, epidermal growth factor), cytokines (tumour necrosis factor-α, fas ligand, Rantes), protein kinase C, ADP-ribosylation factor (Arf) family member, Rho family members, cell surface receptors (e.g. antigen, IgG receptor), extracellular matrix (collagen, fibronectin), drugs and physical stimuli.
Molecular biological approaches concluded that PLD is a membrane-associated enzyme, and its activities have been reported for diverse subcellular organelles including plasma membrane, Golgi vesicles, endoplasmic reticulum, secretary vesicles and nuclear membranes. The major question remains—learning more about its localization, identification, structure and functions of different isoenzymes and its regulatory properties, detail of molecular mechanism of regulation and role of protein kinase C, Rho proteins, ADP-ribosylation factor, G-proteins and Ca2+ in each subcellular locations and finally the physiological functions of PLD need to be clarified.
Assay Methods of Phospholipases
Several accurate phospholipase assay methods are known, but a rapid and reliable method for high-throughput screening is still a challenge for efficient detection of superior phospholipases and their practical applications. Some remarkable methods for high-throughput screening of enantioselective biocatalysts particularly lipase have recently been developed using GC and HPLC chromatography, mass spectrometry, IR thermography and circular dichroism analysis [128]. A proper instrumental configuration for high-throughput methodology will also be required for effective detection and screening of lipase/phospholipase-catalysed reactions. In practice, assay methods (Table 4) for screening and measuring different class of phospholipase activities in a reliable manner are discussed in the paragraphs below.
Plate assay methods are most commonly used for microbial cell-based primary screening of phospholipase activity. Estimation of total phospholipase activity in less quantitative manner can be made using an agar diffusion assay based on the plate assay [129]. Eight-millimetre-diameter wells were cut into 20 ml diffusion plates containing 0.5% (w/v) soybean phospholipids, 50 mM/l CaCl2 and 1% (w/v) agar. Then 150-μl filter-sterilized culture supernatant was added into wells. Plates were incubated at 37 °C for 4 days, and diameter of white zone of precipitation was measured [130]. Ghannoun [4] described a plate method for the detection of phospholipase activity in 30 species of Candida, where egg yolk incorporated in dextrose agar-based medium. When grown on this medium, phospholipase-positive Candida isolates produce a distinct dense white zone of precipitation around the colony. The white zone was due the formation of calcium complex with the fatty acids released by the action of phospholipase on the phospholipids present in soybean and egg yolk. These assay plate methods are limited to screen only the extracellular phospholipase activity of any microbe. Soybean and egg yolk contain substrates for both phospholipase (phospholipids) and lipases (triglycerides) which makes the assay non-specific. Furthermore, the assay is not suitable for the screening of microbial isolates that produce low levels of phospholipids. Conformation of phospholipase activity necessitates the use of a specific radiometric or colorimetric assay (Table 4) or fast atom bombardment–mass spectrophotometer (FAB-MS) and the use of concentrated cultrate filtrate, particularly in poorly phospholipase-producing strains.
The PLA1, PLA2 and PLC producing microorganisms can be screened, but PLD activity on CaCl2-agar medium cannot be detected. A colorimetric method was developed for detecting PLD activity on solid media using phosphatidyl-2-naphthol as substrate [131]. In this method, the synthetic substrate phosphatidyl-2-naphthol was hydrolyzed by the action of PLD and released 2-naphthol and phosphoric acid. The liberated 2-naphthol was spontaneously coupled with a coexisting diazonium salt (fast blue B salt) and generated coloured azo dye. The generated azo dye was water insoluble; thus, the positions of the enzyme on solid materials such as nitrocellulose membrane, polyacrylamide gel and agar media can be visualized as coloured bands or spots The water-insoluble azo dye was generated from the 2-napthol liberated by PLD, and the colour of azo dye was stable on a nitrocellulose membrane, polyacrylamide gel and agar. Determination of phospholipase activity by a colorimetric assay using pH indicator is well-known screening method. The pH-stat assay using egg yolk lecithin can measure PLA1 and PLA2 activities quantitatively by titration of the acid products released with time by adding NaOH to maintain a constant pH value [132]. Although it was less quantitative method, it can be more suitable for high-throughput screening by adding a proper instrumental configuration such as autotitrator, autoburette, data recorder etc.
The enzyme activities of PLB, lysophospholipase (LPL) and lysophospholipase–transacylase (LPTA) by applying radiometric analysis using thin-layer chromatography (TLC) and NMR spectroscopy were carried out by Cox et al. [133]. The determination of PLB activity, carriers dipalmitoyl phosphatidylcholine (DPPC; 102 nmol) and 1, 2-di-[1-14C] palmitoyl-phosphatidylcholine (20,000 dpm) was dried under nitrogen and resuspended in 125 mM imidazole-acetate buffer, pH 4.0(assay buffer), by sonication. After addition of culture filtrate (1 to 2 μg of total protein) and incubated for 22 min, the rate of loss of radiolabelled PC was measured. Both LPL and LPTA activities were measured simultaneously with carrier lyso-PC and radiolabelled 1-[1-14C] palmitoyl-lyso-PC (25,000 dpm) suspended in assay buffer. The reaction was carried out at 37 °C for 15 s by adding enzyme solution give a final volume of 0.125 ml. All the reactions were terminated by adding 1.0 ml of chloroform/methanol (2:1, v/v). Reaction products were extracted, separated by TLC and quantitated. LPL activity was measured by the rate of loss of 1-[1-14C] palmitoyl-lyso-PC with release of radiolabelled fatty acids. LPTA activity was estimated by the rate of formation radiolabelled DPPC from radiolabelled 1-[1-14C] palmitoyl-lyso-PC. Phospholipids with 3H-, 14C- and 32P-labelled fatty acids are commercially available for radioisotope assay procedures. Although the assay has routinely been used as highly sensitive and reliable method, it has some drawbacks such as discontinuous, expensive, time and labour-consuming extraction and separation procedures as well as regulations regarding the use of hazardous radio isotopes chemicals.
The radioactive TLC assay [142] using phosphatidylcholine as substrate was commonly used for determination of PLD activity in vitro. The assay involved incubation with a radiolabelled substrate and lipid extraction followed by thin-layer chromatography in order to separate and quantify substrate and product(s). However, the separation principle was not applicable in evaluating anionic substrate phospholipids (like PE, PG, PS and NAPE), NAPE-hydrolyzing PLD activity [143] which produced two lipophilic products, N-acyl ethanolamine (NAE) and PA. Therefore, they developed a rapid assay for the routine detection of NAPE-hydrolyzing PLD activity based on precipitation of radiolabelled substrate (NAPE) in the presence of ZrOCl2, followed by quantification of radiolabelled NAE released into a methanolic supernatant. These assays were discontinuous, expensive and not appropriate when careful kinetics was required.
The spectrophotometric thiol assay was often used as more sensitive and convenient assay for PLA1, PLA2 and PLC [144]. The method of Cho and Kezdy [135] was originally used to determine PLA2 activity with synthetic substrate 4-nitro-3-octanoyloxybenzoic acid. However, Birch et al. [70, 71] showed that it also acts as a substrate for phospholipase B (phospholipid acyl hydrolase activity) and measured phospholipid acyl hydrolase activity, i.e. combination of both phospholipase A and B (Table 4). Jiménez et al. [136] developed a continuous spectrophotometric method for assaying PLA2 activity. The assay was based on a coupled enzymatic assay, using dilinoleoyl phosphatidylcholine as substrate and lipoxygenase as coupling enzyme. The linoleic acid released by PLA2 was oxidized by lipoxygenase, and then, PLA2 activity was measured spectrophotometrically at 234 nm. The increase in absorbance is due to formation of the corresponding hydroperoxide from the linoleic acid. The preparation of phosphatidyl-p-nitrophenol was used as a substrate in assay for the quantitative evaluation of phospholipase D catalysed hydrolytic activity [145]. The method is rapid, accurate and inexpensive. The trans-phosphatidylation activity of PLD enzyme was assayed as specific spectrometric method, in which p-nitrophenol liberated by PLD-catalysed reaction of phosphatidyl-p-nitrophenol and ethanol in an aqueous-organic emulsion system [146]. The most frequently reported spectrophotometric assay for PLC activity is using p-nitrophenyl phosphorylcholine as substrate liberated p-nitrophenol was measured at 410 nm [39, 134]. An improved assay for PLC activity was described by Durban and Bornscheuer [137] in which the liberated phosphate compound was cleaved with an alkaline phosphatase. The phosphate formed was then extracted with n-butanol and reduced by an acidic SnCl2 solution. The phosphate was converted to phosphomolybdate complex and quantified by spectrophotometric measurement at 700 nm. The method was demonstrated for concentration range from 10 nM to 10 mM with different head group phospholipids in an aqueous and a two-phase system. This method has additional advantage that crude enzyme can be used without the purification.
The sensitive and continuous fluorescence-based assays are applicable to all types of phospholipases with availability of suitable substrates and instrumentation. This can be readily applied to high-throughput screening for kinetic analysis and inhibitor discovery [138]. The assays based on change in the properties of the probe as the substrate was converted to product. Pyrene, Bis-BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-S-indacene), coumarin, dansyl, NBD-hexanonyl and naphthyl-labelled phospholipids were used for phospholipases continuous fluorescence assays [139]. A convenient and specific fluorescent turnoff assay for PLC was developed and applied with additional advantages of using natural lipid substrates [140]. The assay principle is based on the reversible interaction between the natural substrate (PC) and a fluorescent (water soluble conjugated polyelectrolyte, CPE). The fluorescent intensity of the CPE in water was increased substantially by the addition of the phospholipid due to the formation of a CPE–phospholipid complex. Addition of the enzyme PLC caused decrease in the fluorescence intensity (turnoff sensor) due to PLC-catalysed hydrolysis of the PC, which effectively disrupts the CPE–phospholipid complex. The assay has micromolar to 1 nM analytical detection range for PLC.
Spectrophotometric endpoint assay do not permit continuous monitoring of phospholipase activation in live cells. Feng et al. [138] proposed the synthesis as well as the in vitro and in situ evaluation of a novel PC analogue that allowed monitoring of PLA2 activity via sensitive fluorogenic assay. The fluorogenic analogue of PLA2 substrate named Dabcyl-BODIPY-PC or simply DBPC (Dabcyl, 4-4-N,N-dimethylamino phenylazo benzoic acid) was synthesized with a fluorescence quencher, in the sn-1 acyl chain and a BODIPY fluor in the sn-2 acyl chain. PLA2 recognized DBPC and quantifiable fluorescence enhancement of DBPC occurred upon PLA2 hydrolysis. Thus, DBPC can be used as a sensitive fluorogenic probe for in vitro screening for drug discovery and subcellular localization of enzyme activity and would expedite studies of PLA2 in cellular signalling.
FAB-MS had been applied to the study of bacterial phospholipids for chemotaxonomic purposes, and the technique provides useful information about phospholipids and fatty acids and can be applied to complex mixture of these compounds [70]. The negative-ion FAB-MS was used to monitor the breakdown of soybean phospholipids by extracellular phospholipase activities of Aspergillus fumigatus. Assay of PLA2 using scattering mode of a spectrofluorimeter avoids time-consuming and expensive fluorescence-labelled reagents. The hydrolysis of dimyristoyl phosphatidylcholine by snake venom PLA2 was monitored by spectrofluorimeter at 650 nm [147]. The detection sensitivity by the scattering mode was double when compared to the titrimetric assay.
A bioluminescent assay for detection of PLC activity using bioluminescent marine bacteria was developed by Cho et al. [141]. The substrate sn-1,2-dimyristoyl phosphatidylcholine and PLC reaction product sn-1,2-dimyristoyl glycerol was further hydrolyzed with lipase. The released myristic acid was quantified directly using the dark mutant of Vibrio harveyi.
Industrial Applications of Phospholipases
Phospholipase of the invention could be used in various industrial applications of phospholipases, e.g. use in oil refining, dairy, baking, health food industries etc. PLC and PLD were chiefly used for pharmaceutical, medical and analytical purposes. The microbially produced PLD was mainly discussed for the synthesis of chemicals by trans-phosphatidylation, i.e. exchange of the alcohol moiety attached to the phosphatidic acid residue. PLA2 were reported to be suitable for enzymatic degumming of edible oils and synthesis of triglycerides enriched in polyunsaturated fatty acids (PUFAs). Mammal, plant or microbially produced phospholipases are being sold for these purposes, but the uses of microbial enzymes are cost-effective and environmental friendly.
Use in Health Food Industries
Preparation of Highly Unsaturated Fatty Acids
There has been an increasing awareness in recent years of the nutritional importance of certain fatty acids such as γ-linolenic acid, arachidonic acid and docosahexaenoic acid (DHA). These occur naturally but often at low levels along with other acids. There is a demand of higher levels of these acids in industries and preparation of pure material for research purpose. While such material can be produced by conventional physical and chemical methods of separation, these are not always appropriate if the material is subsequently to be used for nutritional studies. The problem has been successfully overcome by exploiting the specificity of enzymes. Phospholipase A2 was used to prepare phosphatidylcholine enriched with n3PUFA [6]. Phosphatidylcholine from egg yolk was hydrolyzed to lysophophatidylcholine (30 °C, 16 h) and re-acylated with a PUFA-enriched mixture of acids containing eicosapentaenoic acid (EPA) 41% and DHA 30%. The phosphatidylcholine finally contained 16% and 11%, respectively, of these two acids. Using a 90% concentrate of DHA, phosphatidylcholine with 35% of DHA was prepared [148].
Pig pancreas phospholipase A2 was used to mediate trans-esterification of soy lecithin with highly unsaturated fatty acids (at sn2 position) to produce a lecithin. The lecithin could be used for pharmaceutical, nutritional and industrial applications [149–152]. For the trans-esterification, 14 μmol soy PC was combined with 60 μmol of acyl donors (EPA, DHA, EPA ethyl ester or DHA ethyl ester) and 0.55 g glycerol in a screw-cap vial at 600–800 rpm and 25 °C under Argon gas atmosphere. The products of the reaction were treated with chloroform/methanol/water (10:5:3 v/v/v) and separated in a separator funnel. The chloroform layer with the reaction products was concentrated and applied to silica gel TLC plate and developed with chloroform/methanol/water (65:25:4 v/v/v). The lecithin fraction was scraped off and eluted with methanol. As a result, 70% of the total fatty acid at sn2 was incorporated as highly unsaturated fatty acid into soy lecithin. Maruha [152] has developed a method for production of glyceride containing arachidonic acid in high concentration (e.g. 16.5%) by phospholipase A2. It involved yolk phospholipase hydrolysis, fatty acid extraction and glycerol addition to the fatty acid.
Functional Food Manufacturing
Functional foods are food products that provide a health benefit over and above their basic nutritional value. Degussa food ingredients has a range of phosphatidylserine that was derived from soy in process using trans-esterification (trans-phosphatidylation) reaction of PLD [153]. It is also possible to synthesize naturally occurring low abundance phospholipid such as phosphatidylethanolamine, phosphatidylserine and phosphatidylglycerol from phosphatidylcholine a high abundant phospholipid by PLD [154].
Cholesterol Reduction in Food
A method of reducing the cholesterol content of a food involves subjecting the food to either an enzymatic treatment (especially with cholesterol oxidase (EC 1.1 3.6) or a cholesterol oxidase producing microorganism) or to biochemical treatment (degradation of cholesterol) [155]. The treatment with hydrolytic enzyme and the biochemical treatment may be performed simultaneously. The food usually is egg yolk, meat, fish or dairy products. The biochemical, chemical and/or physical treatment of the food to obtain the cholesterol-reduced product was improved by treating it with a hydrolytic enzyme, e.g. phospholipase A1, phospholipase A2, lysophospholipase phospholipase B or phospholipase D (but not phospholipase C) as an alternative to cholesterol oxidase treatment.
Removal of Mucilage and Content of Phosphorus Component or Degumming of Plant Oil
Vegetable oil contains phospholipids (commonly known as gums), which have a negative impact on the storage stability and downstream processing of the oil. In oil refining industries, PLs have been used for removal of mucilage and content of phosphorus component in crude vegetable oil. It is carried out by PLA1, PLA2 or PLB but not with PLC or PLD [156–161].
The phospholipase enzymatic treatment process is applicable to purification of any edible oil which contains phospholipid, e.g. vegetable oil such as soy bean oil, rape seed oil and sunflower oil [157–159]. The process for reducing the content of phospholipid in edible oil comprises of two steps, treating the oil with phospholipase (A1, A2 or B) to hydrolyze the phospholipids such as lecithin and other accompanying hydrophilic components and separating an aqueous phase containing the hydrolyzed phospholipids from the oil. The process was named ‘wet refining to remove mucilage’ as it was carried out by extraction with water. A part of phosphotides may be left in the oil through above treatment and are termed as ‘non-hydrolic phosphotides’ (NHP). NHP mainly consist of lysophospholipids and phosphatidic acids and/or calcium and magnesium salts. The NHP content should be removed for production of edible oils, and the remaining phosphorus content should not exceed to 5 ppm [157]. One litre of soybean oil was processed for ‘wet refining to remove mucilage’. The oil containing 130 ppm of residual phosphorus was heated to 50 °C in a Florence flask; 0.1 g of a pure phospholipase A2 (an activity of 10,000 U/g/l of phospholipase A2 unit liberates 1 μmol of fatty acid per minute from egg yolk at 40 °C and pH 8), 1 g of sodium citrate and 20 g of sodium dodecyl sulphate were dissolved in 33.3 g of water, and the solution was emulsified in the oil to form droplets 0.1 μm in diameter using an external centrifugal pump. After treatment for 3 h, a sample removed by centrifugation was found to have an NHP content of 34 ppm of phosphorus. After increasing the temperature to 75 °C and continuing the treatment for one further hour, the NHP content has decreased to 3 ppm of phosphorus. The oil which had thus been treated can be now subjected to physical refining. The microbial origin (e.g. Aspergillus sp.) phospholipase A1, A2 and B were used for reducing content of phosphorus in plant oils [115, 162, 163].
Use in Mayonnaise Preparations
PLA2 was used for enzymatic hydrolysis of egg yolk to give better emulsion in mayonnaise preparations [164]. Enhancement of interaction between proteins and phospholipids may be the reason why PLA2 increases emulsion stability. Structural and functional changes of low density lipoprotein were observed in PLA2-treated hen egg yolk [165].
Use in Bread Making
Lipases and phospholipases are used in baking industry since 1990. A phospholipase from Fusarium oxysporum with both lipase and phospholipase activity was introduced to the market by novozymes A/S for baking application under the trade name Lipopan F™. The value of lipases in bread making lies in their ability to increase loft volume and improved softness. Phospholipase and galactolipase activities were shown to provide even better emulsification in the dough [158, 166, 167]. Lipase/phospholipase baking has optimal activity on the different substrate available in flour such as triglycerides/phospholipids/galactolipids and giving the optimal gas bubble stability. The phospholipases are able to replace emulsifiers such as diacetyl tartaric acid ester of monoglycerides and sodium steroyl lactylate [7].
Use as Animal Feed Additives
A process for the production of animal feed comprises mixing the phospholipase and at least one phospholipid with feed substances. The composition contains a phospholipid, especially lecithin and PLA2 [168]. Phospholipase A2 can be obtained from mammals, plants, microorganisms and/or derived from cattle, pig, mouse, rat or humans. It can also be produced by expression of recombinant DNA in a host cell, especially Escherichia coli, S. cerevisiae and Aspergillus niger. The composition improves lipid digestibility and promotes feed utilization efficiency for feed of non-ruminating animals especially calves.
Use in Synthesis of Surfactants
A surfactant composition contains at least one lysophospholipid, where one of the aliphatic acyl group (R′ and R″) and the H was other an aliphatic acyl group, and X was a residue formed by removal of H from a hydroxyl group of polyhydric alcohol (e.g. ethylene glycol, glycerol, sorbitol, mannitol, glucose, galactose, fructose, sucrose or lactose). Partial hydrolysis of lysophospholipid, was performed using a lipase (EC 3.1 1.3) or preferably PLA2 (EC 3.1.1.4) at pH 7.0–8.5 in the presence of calcium ions. The composition was a water soluble and efficient surfactant [169].
Preparation of Pork Meat Biosensor
A novel time–temperature indicator based on a pig pancreas PLA2–phospholipid system was developed to monitor quality change of frozen food during storage [170].
Use in Detergent
The detergent composition using phospholipase variant was formulated for hand or machine dishwashing operations, e.g. as described in GB 2,247,025 (Unilever), WO1998/26057 and WO 99/01531 (Procter & Gamble) [158]. In a dishwashing composition, the variant might be effective for removal of greasy/oily stains, for prevention of the staining/discoloration of the dishware and/or plastic components of the dishwasher by highly coloured components and the avoidance of lime soap deposits on the dishware. It was also used as hand or machine laundry detergent compositions, the variant may be effective for the removal of fatty stains, for whiteness maintenance and for dingy cleanup [166].
Use in Dairy Industries
Maximizing cheese yield without compromising product quality is a key concern for industrial cheese manufacturers. Normally 85–95% milk fat and 75% of milk protein are entrapped in the cheese curd. The rest is lost in whey and brine [171, 172]. An enzymatic method for increasing cheese yield using phospholipase was patented by Nielsen [173].
The hydrolysis of milk phospholipids with PLA1 from Fusarium venenatum significantly increased the cheese yield through better moisture and fat retention during whey drainage and stretching. The fungal PLA1 from F. venenatum hydrolyzed the major milk phospholipids PE and PC. The phospholipase A1 was used to decrease the oiling-off effect in cheese and to increase cheese yield. The oiling-off effect is the tendency of the cheese to form free oil upon storage and/or melting. A suitable dosage of phospholipase will usually be in the range 0.001–0.5 mg enzyme protein per gram milk fat. They manufactured low-moisture mozzarella cheese as an ingredient in food, for instance in pizza, where it is most important functionality was the melting behaviour. They evaluated the flow ability, stretch ability and browning of the phospholipase cheeses [174].
Lipid Second Messengers and Phospholipases
Genetic and pharmacological approaches implicated PLD for control of intracellular membrane transport and recognition of the actin cytoskeleton [121]. The PLD activity leads to formation of phosphatidic acid included in phosphatidylinositol-4 phosphate-5-kinases and the RAF protein kinase. Phosphatidic acid can also be converted to two other lipid mediators, diacylglycerol and lysophosphatidic acid. Regulation of PLD activity is complex, and studies in this area have been further complicated by the lack of good antibodies, purified enzymes and the existence of several isoforms. Mammalian PLD activity was highly regulated by the large number of factors like fatty acids, phosphoinositol, small GTP binding proteins, protein kinase C, Ca2+, phosphorylation and other negative regulators. Many of these factors regulate PLD activation in a synergistic or antagonistic manner. Phospholipase D generates PA which subsequently can be either metabolized by PLA2 generating lysophosphatidic acid, a potent cellular mitogen, or phosphatidate phosphohydrolase yielding DAG. Sphingomyelinase, a phospholipase C-type enzyme, and related enzymes of sphingolipid metabolism were implicated in apoptosis and other signalling processes.
Other important phospholipases include the phosphatidylinositol-specific PLC, which controls the production of inositol-1,4,5-triphosphate (second messenger) and initiates the signal transduction cascade in response to cell surface receptor activation [117, 118]. C-type phospholipases catalyses the cleavage of membrane phospholipids to 1,2-diacylglycerol and the respective phosphoryl compounds. In eukaryotic cells, activation of PLC frequently occurs through surface receptor stimulation. PLC specific for phosphatidylinositol and its phosphorylated derivatives have been studied extensively [121]. 1,2-Diacylglycerol acts as second messenger in cellular signalling through activation of protein kinase C.
Numerous signal transduction processes involved lipids as signalling molecules. Many of those molecules generated by phospholipases such as PLA2 released fatty acids (arachidonic acid and lysophospholipids). Each of those products was implicated in signal transduction processes and also served as a precursor for eicosanoids including prostaglandins, leukotrienes and lipoxins or PAF [175]. These compounds were implicated in numerous inflammatory diseases such as rheumatoid arthritis, sepsis, intestinal bowel disease, asthma as well as playing a role in cancer, atherosclerosis and premature parturition. In summary, the phospholipases generate numerous lipid products which control much of cellular signalling. Although the importance of these enzymes in eicosanoid metabolism signal translation is not questioned, much remains to be studied regarding the regulation of these enzymes and their pathophysiological roles.
Potential Role of Phospholipase as Virulence and Pathogenicity Factor
Microbial pathogens use a number of genetic strategies to invade the host cell and cause infection. Invasion of host cells by microbes entails penetration and damage of the outer cell wall. The transmigration process is mediated by either physical or enzymatic means; phospholipases and proteinases are likely to be involved in the membrane disruption processes that occur during host cell invasion. Destruction of phospholipids, by microbial extracellular PLs and subsequent changes of membrane constituents which lead to cell damage is regarded as major virulence mechanism in infection. Table 2 summarizes the major sources of phospholipases: its involvement in pathogenesis and the substrate specificity for each class of PL.
The phospholipases have been included among the virulence and pathogenicity factor that damage host cells [105, 106]. Evidence implicating phospholipases in host cell penetration, cell lysis and injury by bacteria and fungus have been reported for Rickettsia rickettsii [94], Clostridium perfringens [12], Legionella pneumophila [38–40], Helicobacter pylori [55], Yersinia enterocolitica [58], Candida albicans [4], Cryptococcus neoformans [67, 68] and A. fumigatus [70].
Bacterial Phospholipase and Pathogenesis
Extracellular phospholipase has been implicated as pathogenesis of disease for bacteria was the subject of some reviews (Istivan and Coloe [105], Schmiel et al. [57], Songer [176], Titball [177]). These reviews have focused on disease properties and the best characterized bacterial phospholipase virulence factors which were mostly PLC and later PLA. We wished to increase the scope of review in terms of the types of phospholipases covered. Some examples of bacteria phospholipase their potential role in virulence and pathogenicity are described here.
PLCs were the best studied bacterial phospholipase produced by the species of Clostridia, Bacillus or Listeria that were zinc-dependent metalloenzyme with molecular mass in the range 29–43 kDa. C. perfringens produces α toxin or lecithinase, i.e. a PLC that damages the membranes of erythrocytes and tissue cells. As in tetanus, gas gangrene results from contamination of wounds, by above bacteria. The bacteria C. perfringens is highly invasive and spreads rapidly through tissues. Awad et al. [178] studies the pathogenesis of C. perfringens-mediated gas gangrene. Their study provided definitive genetic evidence of the essential role of PLC in gas gangrene or clostridial myonecrosis. They used reverse genetics to construct a suicide plasmid in which the ple gene encoding PLC was inactive. Using this plasmid, they isolated mutant that had lost their ability to produce detectable PLC. Comparing the mutants and their parent in a mouse virulence model showed that the mutants were markedly lass pathogenic than the parent strain. Animals infected with the phospholipase-deficient mutants showed minimal swelling, less muscle destruction, inflammation or necrosis of the infected tissues and remained active with a healthy appearance. Animals challenged with the parent strain showed extensive swelling of the infected foot, leg and lips as well as demonstrable signs of haematuria and extensive muscle destruction. The precise mode of action of α toxin is not yet certain, but the destructive effects were resulting from products of PLC activity. Incubation with purified α toxin was demonstrated to induce expression of cytokines TNF, αPAF and IL-8. In addition, α toxin activity was shown to trigger the arachidonic acid cascade which leads to cytokine release and inflammation [179]. Williamson and Titball produced genetically engineered vaccine against α-toxin of C. perfringens, which protects mice against experimental gas gangrene [180].
L. monocytogenes [16–18, 181] secretes two extracellular PLCs: (a) inosital-specific PLC (PI-PLC) and (b) PC-PLC. PC-PLC was broadly active PLC with the ability to hydrolyze macrophages, fibroblast and epithelial cell phospholipids. The zinc-metallo-PLC was active towards PC, but individual enzymes have different activities towards other phospholipids and sphingomyelin [177]. These two PLCs induced ceramide generation (product of PLC hydrolysis of sphingomyelin), nuclear factor-KB activation and E-selectin expression in human epithelial cells which contribute to immune modulation and the pathogenesis of L. monocytogenes [19].
Several PLCs including phosphatidylinositol-specific PLC, PC preferring, as well as sphingomyelinase were produced by B. cereus [27–29]. The contribution of PLs to the virulence of B. cereus has not been investigated in animals, and PLC of this bacterium was considered to be nontoxic. The PC-preferring PL and the sphingomyelinase were active in cancer to cause haemolysis [182]. Wazny et al. [183] reported that B. cereus strains producing PLC caused degranulation of human neutrophils with dose-dependent release tissue damage and suggested that B. cereus protects itself against phagocytosis by releasing PLs.
The phospholipase A activity has been identified in a number of pathogenic bacteria, but its role in virulence is yet to be fully elucidated. However, evidence suggests the PLA activity has a significant role in pathogenesis of Rickettsia prowazekii [34, 35]. The demonstration of host cells exposed to large number of Rickettsia species release considerable amounts of lysophospholipids and free fatty acids into the culture medium. Silverman et al. [33] confirmed these findings and provided suggestive evidence that phospholipase activity penetrates the host cells. H. pylori possess several different phospholipase activities such as PLA1, PLA2 and PLC. These were linked to the degradation of the phospholipids components of the mucosal barrier and were able to initiate production of leukotrienes and eicosanoids from arachidonic acid. This can also affect membrane permeability and mucous discharge [55]. Moreover, the pH-activated PLA2 in H. pylori acted as an initial mucosal barrier breaker in pathogenesis of peptic ulcer [56]. The research indicated that the gastric juice of H. pylori-infected individuals contained significantly higher PLA2 and PLC concentrations than those of healthy individuals.
The PLC activities in several Legionella were associated with pneumonia, destruction of lung surfactant and epithelial cells [38]. Flieger et al. [39] reported two PLA activities (PLA and lysophospholipase) in L. pneumophila and considered them as powerful agents in the mediation of pathogenicity due to destruction of lung surfactant and epithelial cells. PLA activity was secreted prior to lysophospholipase activity which led to accumulation of the cytotoxic agent [40].
The extracellular PLD of C. pseudotuberculosis [36] in caseous lymphadenitis of small ruminants played role in pathogenesis. C. pseudotuberculosis PLD predominantly found in the secreted fraction degraded sphingomyelin and lysophosphatidylcholine (product of PLA hydrolysis of PC). Purified PLD was dermonecrotic and lethal when injected into animals [184]. PLD-toxoid vaccines gave significant protection against caseous lymphadenitis in sheep, supporting the role of PLD in tissue destruction either directly or indirectly. The gene encoding PLD in C. pseudotuberculosis has been cloned and sequenced [37]. Targeted mutagenesis of PLD reduced the ability of the bacterium to establish a primary infection [36].
There are some reports of PLB produced by pathogenic bacteria such as Moraxella bovis [1]. However, there is no evidence that these enzymes are toxic or play role in virulence from bacteria.
Fungal Phospholipase and Their Potential Role in Virulence
Studies on fungal phospholipases have mainly focused on the characterization of PLB from non-pathogenic fungi, including S. cerevisiae [74, 75], Torulaspora delbrueckii [79], Schizosaccharomyces pombe [78] and Penicillium notatum [76, 77]. Of the medically important fungi, extracellular phospholipase activities have been described in C. albicans [4], A. fumigatus [70] and C. neoformans [67]. Fungi possess a broad range of hydrolytic enzymes that attack neutral lipid and phospholipids especially during infection of mammalian host. PLA2 and PLB enzymes released by fungi can play important roles not only for nutrient acquisition and tissue invasion but also for intricate modulation of the host’s immune response.
Extracellular phospholipases as virulence factor in C. albicans pathogenesis were proven [4]. C. albicans causes candidiasis of nail, skin and mucous membranes and are superficial mycoses or dermatomycoses type of fungal disease. The fungus invades subcutaneous tissues or the lungs from which they spread to other organs of the body, become established and produce disease. Infection with C. albicans in mouse also caused a mycotic endocarditis, pulmonary moniliasis and virginities. In the patients with impaired immunity due to cancer or AIDS, the incidence of fungal infections has risen dramatically. C. albicans was most commonly isolated pathogen from nosocomial blood stream infection in the USA, and oral candidiasis was one of the first indicators of HIV disease progression [60]. Samaranoyake et al. [61] screened 41 Candida isolates for PL activity by using plate assay method and found that 79% of the C. albicans strains tested produced extracellular PLs. Ibrahim et al. [62] carried out a comparative study in vitro on 22 isolates of C. albicans (11 isolates were obtained from patient with disseminated candidiasis and 11 were commensal isolates recovered from the oral cavities of healthy volunteers) by using egg yolk agar-based method. They found significant higher levels of PL production in the blood isolates then in the commensals. In the same study, they examined nine blood isolates for expression of virulence factors like phospholipase and proteinase production, adherence, germination, growth rate and ability to damage endothelial cell. The high phospholipase production was correlated with increased virulence in mouse model of disseminated candidiasis. To obtain further evidence of the contribution of PLs to candidal pathogenicity, the cloning of two genes encoding PLB (caPLB1 and caPLB2) and one gene (caPLD) coding for PLD was carried out [63, 64]. Gene disruption/deletion is frequently used to determine the functionality of a specific gene. A number of genes were disrupted to evaluate their contribution to the virulence of C. albicans [4, 155]. PLB-deficient mutants were constructed by targeted gene disruption using the URA3-blaster technique, which reduced the ability of C. albicans to secrete the enzyme [65]. Mouse models for haematogenous dissemination candidiasis as well as an infant mouse oral intragastric infection model were used to determine the role of caPLB1 in virulence of C. albicans [66]. In haematogenous dissemination model, 5 × 105 cells were intravenously injected in both parent type and the PLB-deficient mutant strain separately. Parent strain model caused infection in 9 days whereas no infection was found with PLB-deficient mutant till 15 days. In the experiments with oral intragastric infant mouse model, the infant mice were inoculated with 2 × 108 blastospore. Liver and kidney colonization for parental strain was 90% and 70%, respectively, whereas the liver and kidney colonization for caPLB1-deficient mutant was 45% and 27%, respectively.
Two additional genes were isolated for PLB (caPLB3 and caPLB4) and another gene caPLB5 encodes PLA2 [105]. caPLB5 null mutants were constructed in a wild-type strain of C. albicans. Phenotypic analysis of the caPLB5 null mutants revealed loss of cell-associated PLA2 activity and significant decrease in liver colonization in an experimental mouse model for disseminated candidiasis. Thus, C. albicans has both PLA2 and PLB activities involved in pathogenesis and may have high degree of regulation and functional diversity.
A. fumigatus is a human opportunistic fungal pathogen causing a wide range of diseases including allergic, bronchopulmonary aspergillosis and invasive aspergillosis mainly in immunosuppressed patients. Several pathogenicity determinants for virulence of Aspergillus species have been suggested. Some of these are products of elastase, ribotoxin and other extracellular toxins. There are no specific virulence factors which have been determined. Birch et al. [70] demonstrated the production of extracellular PLC activity in A. fumigatus by using FAB-MS. Further the same group compared clinical and environmental isolates of A. fumigatus for extracellular PLC and a phospholipids acyl hydrolase (PLA and/or PLB) activity. Clinical isolates of A. fumigatus produced the largest zone size in a diffusion assay and more extracellular PLC than environmental isolates. However, environmental isolates of A. fumigatus showed increased acyl hydrolase activity compared to clinical isolates. The study suggested that PLC activity were important in the pathogenicity A. fumigatus. Three PLB genes (afplb1, afplb2, afplb3) from A. fumigatus have been reported using degenerated primer in PCR amplification [72]. The influence of lecithin on the expression of these genes was investigated and found that gene transcripts of afplb1 and afplb3 were upregulated by lecithin (respectively 5-fold and 300-fold) whereas afplb2 expression was not affected. Lecithin (dipalmitoyl phosphatidylcholine) was major component of lung surfactant, and lungs were the main portal of entry for A. fumigatus, therefore indicating that the PLBs were potentially involved in the pathogenesis. The extracellular PLBs (Afplb1 and Afplb3) and PLC activities as virulence factor in cell biology of A. fumigatus remains to be tested. This may be important in the interaction of A. fumigatus with host cell membranes.
Chen et al. [67, 68, 116] examined 50 C. neoformans isolates for extracellular PL activity using an egg yolk-based agar assay. Forty-nine of these isolates produced a pericolonial precipitate indicative of PL activity. Further, they identified enzyme activities in one clinical isolate (strain BL-1 of C. neoformans var. grubii) as PLB, LPL (lysophospholipase) and LPTA (lysophospholipase/transacylase) activities by applying radiometric analysis using TLC and NMR spectroscopy. The enzyme was purified to homogeneity from C. neoformans using (NH4)2SO4 fractionation and hydrophobic interaction, anion exchange and gel filtration chromatography. All three enzyme activities co-purified as a single protein that was an acidic glycoprotein containing N-linked carbohydrate moieties with pI values of 5.5 and 3.5. The enzyme was active only at acidic pH (pH optimum of 4.0 for PLB and 4.0–5.0 for LPL and LPTA). Wright et al. [69] isolated another phospholipase with only LPL and LPTA activities from Cryptococcus gattii and found it to be a 66-kDa protein (by SDS/PAGE). The PI was 6.3 and it was active at high temperatures (to 70 °C) as well as both acidic and neutral pH values.
The phospholipase-encoding gene (PLB1) was cloned from C. neoformans and constructed plb1 mutants using targeted gene disruption. The enzyme activities were markedly reduced in the mutant plb1 strain and were significantly less virulent than the control strains in both the mouse inhalational model and the rabbit meningitis model [67]. They were also able to restore virulence to the plb1 strain by reconstitution of extracellular phospholipase activities and concluded that the PLB1 gene product was virulence factor for C. neoformans in experimental cryptococcosis. The finding provided insight into the pathogenesis of fungal infections and allowed for the potential targeting of extracellular phospholipases in the development of anti-fungal drugs. The same group further showed that anti-cnplb1 antibodies were found in the sera of patients for up to 2 years after cryptococci infections. The potential of PL was demonstrated by Hanel et al. [185] who found increased survival of C. albicans-infected mice when phospholipase inhibitors were given in combination with the anti-fungal fluconazole.
Conclusion and Outlook
The phospholipases produced from mammals, plant or microbes are commercially available for industrial purposes. The use of microbial phospholipases is environmental friendly and cost-effective. The use of thermostable enzymes can improve the properties of industrial phospholipases and provide benefits like increased substrate solubility, decreased viscosity of the medium and a lower risk of microbial contamination. Thus, the search for microbial thermostable phospholipases is yet another area of study. A thermostable PLA1 was created by evolutionary molecular engineering by using a combination of mutagenic PCR and filter-based screening. The thermal stability and catalytic activity of PLA1 from Serratia sp. strain MK1 were successfully improved and had broadened the possibility of the application of this PLA1 in various phospholipid-related industries [186].
The recent progress on virulence and pathogenic factor of phospholipases were derived from studies of phospholipases from pathogenic bacteria to fungi and their hydrolytic functions causing direct tissue damage. The other possible roles of phospholipases in pathogenesis include the liberation of lipid second messengers, such as inositol triphosphate, arachidonic acid, and the lysis of phagocytic cells and compartments [57, 187]. Prostaglandins are potent regulators of host immune response. Enhanced prostaglandin production during fungal infection could be an important factor in promoting fungal colonization and chronic infection. Host cells are one source of prostaglandins. Another potential source of prostaglandins is the fungal pathogen itself. The production of prostaglandins was determined in pathogenic yeasts C. neoformans and C. albicans. The role of prostaglandins in fungal biology and disease pathogenesis was also defined [188]. C. neoformans and C. albicans both secreted prostaglandins de novo or via conversion of exogenous arachidonic acid. Pathogenic fungi produce eicosanoids like prostaglanding or leukotrienes from arachidonic acid. The role of gene cnPLB in eicosanoid production from C. neoformans was investigated and demonstrated that PL was necessary for release of precursor arachidonic acid from arachidonoylphosphatidylcholine [188–191]. The discovery that pathogenic fungi produce and respond to immunomodulatory eicosanoids reveals a virulence mechanism that has potentially great implication for understanding the mechanisms of chronic fungal infection, immune deviation and fungi as disease cofactors. Moreover, the knowledge of phospholipase gene regulation in vivo would be helpful into further understanding of bacterial or fungal infection.
Once the importance phospholipase in bacterial or fungal virulence is proven, the next challenge would be drug discovery: to use these enzymes in vaccine development, to identify, improve and design phospholipase inhibitors and use enzyme as diagnostic marker of infections [192]. Approaches for evaluating the significance of phospholipases as virulence factor for the disease and identification of its inhibitors from different anti-fungal or antibacterial herbs or by chemical inhibitor would be potentially an effective therapy for several inflammatory conditions. PLA2 inhibitors are used as potential anti-inflammatory agents for inhibition of PLA2 activity and are suggested for their therapeutic use [193, 194]. The starting point in seeking new and specific anti-inflammatory drug against such enzymes (PLs) are ellagic acid derivatives, koninginins E and F. Ellagic acid derivatives isolated from Casearia sylvestris. These derivatives are characterized as anti-PLA2 activity [195]. Trichoderma fungi produce substances known as koninginins that have great structural similarity to compounds like flavonoids and vitamin E and are able to inhibit the PLA2. These isolated compounds can be used for medicinal applications, particularly in inflammatory reactions. The isolated koninginins A, E and F from Trichoderma koningii (endophytic fungus) were analysed for capabilities of inhibiting oedema-inducing, myotoxic and enzymatic activities of the total venom of Bothrops jararacussu snake. Koninginins E and F are more efficient in the inhibition of PLA2, and the structure and action mode of these molecules is similar to vitamin E.
Abbreviations
- PL:
-
Phospholipase
- PLA:
-
Phospholipase A
- PLB:
-
Phospholipase B
- PLC:
-
Phospholipase C
- PLD:
-
Phospholipase D
- PA:
-
Phosphoric acid
- PC:
-
Phosphatidylcholine
- PI:
-
Phosphatidylinositol
- PE:
-
Phosphatidylethanolamine
- PG:
-
Phosphatidylglycerol
- SM:
-
Sphingomyelin
- LPC:
-
Lysophasphatidylcholine
- LPI:
-
Lysophosphatidylinositol
- LPG:
-
Lysophosphatidylglycerol
- PLC N:
-
Non-haemolytic PLC
- PLC H:
-
Haemolytic PLC
- DLPC:
-
Dilinoleoyl phosphatidylcholine
- DMPC:
-
1,2-sn-Dimyristoyl phosphatidylcholine
References
Verheij, H., & Dijkstra, B. W. (1994). In P. Wooley & S. V. Petersen (Eds.), Lipases, their structure, biochemistry and application (pp. 119–138). Cambridge: Cambridge University Press.
Farn, J. L., Strugnell, R. A., Hoyne, P. A., Michalski, W. P., & Tennent, J. M. (2001). Journal of Bacteriology, 184, 6717–6720.
Exton, J. H. (1997). The Journal of Biological Chemistry, 272, 15579–15582.
Ghannoun, M. A. (2000). Clinical Microbiology Review, 13, 122–143.
Singer, W. D., Brown, H. A., & Sternweis, P. C. (1997). Annual Review Biological Chemistry, 66, 475–509.
Na, A., Erilksoon, G., Eriksson, S., Osterberg, E., & Holmberg, K. (1990). Journal of the American Oil Chemists’ Society, 67, 766–770.
De Maria, L., Vind, J., Oxenbell, A., Svendsen, S., & Patkar, S. (2007). Applied Microbiology and Biotechnology, 74, 290–300.
Song, J. K., Han, J. J., & Rhee, J. S. (2005). Journal of the American oil Chemists’ Society, 82, 691–705.
Wang, X., Wang, C., Sang, Y., Qin, C., & Welti, R. (2002). Physiologia Plantarum, 115, 331–335.
Hanasaki, K., & Arita, H. (2002). Annual Report of Shionogi Research Laboratories, 52, 1–22.
Leslie, D., Fairweather, N., Pickard, D., Dougan, G., & Kehoe, M. (1989). Molecular Microbiology, 3, 383–392.
Okabe, A., Shimizu, T., & Hayashi, H. (1989). Biochemical and Biophysical Research Communication, 160, 33–39.
Taguchi, R., & Ikezawa, H. (1975). Biochimica et Biophysica Acta, 409, 75–85.
Taguchi, R., & Ikezawa, H. (1978). Archives of Biochemistry and Biophysics, 186, 196–201.
Mengaud, J., Barun-Breton, C., & Cossart, P. (1991). Molecular Microbiology, 5, 367–372.
Vazquez-Boland, J. A., Kocks, C., Dramsi, S., Ohayon, H., Geoffroy, C., Mengaud, J., et al. (1992). Infection and Immunity, 60, 219–230.
Camilli, A., Goldfine, H., & Portnoy, D. A. (1991). The Journal of Experimental Medicine, 173, 751–754.
Marquis, G. A., Jones, H., Johnston, S., Porrnoy, N. C., & Goldfine, D. A. (1995). Infection and Immunity, 63, 4231–4237.
Schwarzer, N., Nost, R., Seybold, J., Parida, S. K., Fuhrmann, O., Krull, M., et al. (1998). Journal of Immunology, 16, 13010–13018.
Berka, R., Gray, G., & Vasil, M. (1981). Infection and Immunity, 34, 1071–1074.
Coleman, K., Dougan, G., & Arbuthnott, J. P. (1983). Journal of Bacteriology, 153, 909–915.
Ostroff, R. M., Vasil, A. I., & Vasil, M. L. (1990). Journal of Bacteriology, 172, 5915–5923.
Vasil, M. L., Krieg, D. P., Kuhns, J. S., Ogle, J. W., Shortridge, V. D., Ostroff, R. M., et al. (1990). Infection and Immunity, 58, 4020–4029.
Projan, S. J., Kornblum, J., Kreiswirth, B., Moghazeh, S. L., Eiser, W., & Novick, R. P. (1989). Nucleic Acids Research, 17, 3305.
Rogolsky, M. (1979). Microbiological Reviews, 43, 320–360.
Doery, H. M., Magnusson, B. J., Gulasekharam, J., & Pearson, J. E. (1965). Journal of General Microbiology, 40, 283–296.
Kuppe, A., Evans, L. M., McMillen, D. A., & Griffith, O. H. (1989). Journal of Bacteriology, 171, 6077–6083.
Johansen, T., Holm, T., Guddal, P. H., Sletten, K., Haugli, F. B., & Little, C. (1988). Gene, 65, 293–304.
Ikezawa, H., Matsushita, M., Tomita, M., & Taguchi, R. (1986). Archives of Biochemistry and Biophysics, 249, 588–595.
Henner, D. J., Yang, M., Chen, E., Hellmiss, R., Rodriguez, H., & Low, M. G. (1988). Nucleic Acids Research, 16, 10383.
Ikezawa, H., Nakabayashi, T., Suzuki, K., Nakajima, M., Taguchi, T., & Taguchi, R. (1983). Journal of Biochemistry, 93, 1717–1719.
Johansen, K. A., Gill, R. E., & Vasin, M. L. (1996). Infection and Immunity, 64, 3259–3266.
Silverman, D. J., Santucci, L. A., Meyers, N., & Sekeyova, Z. (1992). Infection and Immunity, 60, 2733–2740.
Winkler, H., & Miller, E. (1980). Infection and Immunity, 29, 316–321.
Winkler, H. (1986). Annales de l’Institut Pasteur. Microbiology, 137A, 333–336.
McNamara, P. J., Bradley, G. A., & Songer, J. G. (1994). Molecular Microbiology, 12, 921–930.
McNamara, P. J., Cuevas, W. A., & Songer, J. G. (1995). Gene, 156, 113–118.
Baine, W. B. (1988). Journal of General Microbiology, 134, 489–498.
Flieger, A., Gong, S., Faigle, M., Deeg, M., Bartmann, P., & Neumeister, B. (2000). Journal of Bacteriology, 182/5, 1321–1327.
Flieger, A., Gong, S., Faigle, M., Stevanovic, S., Cianciotto, N. P., & Neumeister, B. (2001). Journal of Bacteriology, 183/6, 2121–2124.
Bernheimer, A. W., & Bey, R. F. (1986). Infection and Immunity, 54, 262–264.
De Silva, N. S., & Quinn, P. A. (1987). Journal of Clinical Microbiology, 25, 729–731.
Saxena, M., Gupta, J. K., & Vadehra, D. V. (1989). Folia Microbiology (Praha), 34, 195–201.
Carrea, G. D., Arrigo, P., & Roncaglio, S. (1995). Biochimica et Biophysica Acta, 1255, 273–279.
Seaindo, F., Carrea, G., Arrigi, P., & Servi, S. (1996). Biochemistry, 35, 9631–9636.
Imamura, S., & Horiuti, Y. (1979). Journal of Biochemistry, 85, 79–95.
Nakajima, J., Nakashima, T., Shima, Y., Fukuda, H., & Yamane, T. (1994). Biotechnology and Bioengineering, 44, 1193–1198.
Iwasaki, Y., Nakano, H., & Yamane, T. (1994). Applied Microbiology and Biotechnology, 42, 290–299.
Okawa, Y., & Yamaguchi, T. (1976). Agricultural and Biological Chemistry, 40, 437–438.
Okawa, Y., & Yamaguchi, T. (1976). Agricultural and Biological Chemistry, 40, 277–283.
Armah, D. A., & Mensa-Wilmot, K. (2000). Journal of Biochemistry, 275, 19334–19342.
Nishijima, M., Akamatsu, Y., & Nojimas, P. (1974). Journal of the American Oil Chemists’ Society, 249, 5658–5667.
Doi, O., Ohki, M., & Nojima, S. (1972). Biochimica et Biophysica Acta, 260, 244–258.
de Geus, P., Van Die, I., Bergmans, H., Tammassen, J., & de Haas, G. (1983). Molecular Genetics and Genomics, 190, 150–155.
Nilius, M., & Malfertheiner, P. (1996). Alimentary Pharmacology & Therapeutics, 10, 65–71.
Berstad, A. E., Berstad, K., & Berstad, A. (2002). Journal of Gastroenterology, 37, 738–742.
Schmiel, D. H., Young, G. M., & Miller, V. L. (2000). Journal of Biochemistry, 182, 2314–2320.
Schmiel, D. H., Wagar, E., Karamanou, L., Weeks, D., & Miller, V. L. (1998). Infection and Immunity, 66, 3941–3951.
Mirbod, F., Banno, Y., Ghannoum, M. A., Ibrahim, A. S., Nakashima, S., Kitajima, Y., et al. (1995). Biochimica et Biophysica Acta, 1257, 181–188.
Samaranoyake, Y. H., Dassanoyake, R. S., Jayatilake, J. A., Cheung, B. P. K., Yau, J. Y. Y., Yeung, K. W., et al. (2005). Journal of Medical Microbiology, 54, 583–593.
Samaranayake, L. P., Raeside, J. M., & McFarlane, T. W. (1984). Sabouraudia, 22, 201–207.
Ibrahim, A. S., Mirbod, F., Filler, S. G., Banno, Y., Cole, G. T., Kitajima, Y., et al. (1995). Infection and Immunity, 63, 1993–1998.
Sugiyama, Y., Nakashima, S., Mirbod, F., Kanoh, H., Kitajima, Y., Ghannoum, M. A., et al. (1999). Medical Mycology, 37, 61–67.
Kanoh, H., Nakashima, S., Zhao, Y., Sugiyama, Y., Kitajima, Y., & Nozawa, Y. (1998). Biochimica et Biophysica Acta, 1398, 359–364.
Fonzi, F., & Irwin, M. Y. (1998). Genetics, 134, 717–728.
Leidich, S. D., Ibrahim, A. S., Fu, Y., Koul, A., Jessup, C., Vitullo, J., et al. (1998). The Journal of Biological Chemistry, 273, 26078–26086.
Chen, S., Muller, M., Zhou, Z., Wright, L., & Sorrell, T. (1997). The Journal of Infectious Diseases, 175, 414–420.
Chen, S. C. A., Wright, L. C., Golding, J. C., & Sorrell, T. C. (2000). The Biochemical Journal, 347, 431–439.
Wright, L. C., Payne, J., Santangelo, R. T., Simpanya, M. F., Chen, S. C. A., Widmer, F., et al. (2004). The Biochemical Journal, 348, 377–384.
Birch, M., Denning, D. W., & Robson, G. D. (2004). Medical Mycology, 42/2, 81–86.
Birch, M., Robson, G., Law, D. L., & Denning, D. W. (1996). Infection and Immunity, 64, 751–755.
Shen, D. K., Noodeh, A. D., Kazemi, A., Grillot, R., Robson, G., & Brugeri, J. F. (2004). FEMS Microbiology Letters, 239, 87–93.
Bekkers, A. C., Franken, P. A., Van-Den Bergh, C. J., Verbakel, J. M. A., Verheij, H. M., & De Hass, G. H. (1991). Biochimica et Biophysica Acta, 1089, 345–351.
Merkel, O., Fido, M., Mayr, J. A., Pruger, H., Raab, F., Zandonella, G., et al. (1999). The Journal of Biological Chemistry, 274, 28121–28127.
Rudge, S. A., Zhou, C., & Engebrecht, J. (2002). Genetics, 160, 1353–1361.
Masuda, N., Kitamura, N., & Saito, K. (1991). European Journal of Biochemistry, 202, 783–787.
Saito, K., & Kates, M. (1974). Biochimica et Biophysica Acta, 369, 245–253.
Yang, P., Du, H., Holfman, C. S., & Marcus, S. (2003). Molecular Genetics and Genomics, 269, 116–125.
Kurawabana, Y., Maruyana, M., Watanabe, Y., Tanaka, S., & Tamai, Y. (1988). Journal of Biochemistry, 104, 236–241.
Saffer, L. D., Krug, S. A. L., & Schwartzman, J. D. (1989). The American Journal of Tropical Medicine and Hygiene, 40, 145–149.
Saffer, L. D., & Schwartzman, J. D. (1991). The Journal of Protozoology, 38, 454–460.
Long-Krug, S., Fischer, K., Hysmith, R., & Ravdin, J. (1985). The Journal of Infectious Diseases, 152, 536–541.
Ravdin, J., Murphy, C., Guerrant, R., & Long-Krug, S. (1985). The Journal of Infectious Diseases, 152, 542–549.
Morgan, C. P., Insall, R., Haynes, L., & Cockcroft, S. (2004). The Biochemical Journal, 382, 441–449.
Frank, D., & Gunstone, F. D. (1999). Journal of the Science of Food and Agriculture, 79/12, 1535–1549.
Zhu, H., Dupureur, C. M., Zhang, X., & Tsai, M. D. (1995). The Biochemical Journal, 34, 15307–15324.
Zykle, R. L., & Schremmer, J. M. (1974). The Journal of Biological Chemistry, 249, 1742–1746.
De-Hass, G. H., Bonsen, P. P. M., Pieterson, W. A., & Deenen, M. V. (1971). Biochimica et Biophysica Acta, 239, 252–266.
Kramer, R. M., Hession, C., Johansen, B., Hayes, G., Mc Gray, P., & Chow, E. P. (1989). The Journal of Biological Chemistry, 264, 5768–5775.
Balsinde, J., Diez, E., Schuller, A., & Mollinedo, F. (1986). Phospholipase A2 activity in resting and activated human neutrophils. The Journal of Biological Chemistry, 263, 1929–1936.
Van Den Bosch, H., Aarsman, A. J., & Van Deenen, L. L. M. (1974). Biochimica et Biophysica Acta, 348, 197–209.
Hammond, S. M., Altshuller, Y. M., Sung, T., Rudge, S. A., Rose, K., Engebrecht, J., et al. (1995). The Journal of Biological Chemistry, 270, 29,640–29,643.
Hammond, S. M., Jenco, J. M., Nakashima, S., Cadwallader, K., Gu, Q., Cook, S., et al. (1997). Indian Journal of Biological Chemistry, 272, 3860–3868.
Maury, E., Prerost, M. C., Nauze, M., Redoules, D., Tarroux, R., Charreron, M., et al. (2002). Biochemical and Biophysical Research Communication, 295, 362–369.
Saito, N., & Kanfer, J. (1975). Archives of Biochemistry and Biophysics, 169, 318–323.
Lu, T., Ito, M., Tchoua, U., Takemori, H., Okamoto, M., & Tojo, H. (2001). Biochemistry, 40, 7133–7139.
Maraganore, J. M., & Heinrikson, R. L. (1986). Journal of Biochemistry, 261, 4799–4804.
Chwetzof, S., Tsunasawa, S., Sakiyama, F., Menz, A., & Nigexine, S. (1989). The Journal of Biological Chemistry, 264, 13289–13297.
Reynolds, L. J., & Dennis, E. A. (1991). Methods in Enzymology, 197, 359–365.
Da Silva, S. L., Calgarotto, A. K., Chear, J. S., & Marangoni, S. (2008). Toxicon, 52, 655–666.
Allgyer, T. T., & Wells, M. A. (1979). The Journal of Biological Chemistry, 18, 5348–5353.
Wang, X. (2000). Progress in Lipid Research, 39, 109–149.
Verma, J. N., Bansal, V. S., Khuller, G. K., & Subranianyam, D. (1980). The Indian Journal of Medical Research, 72, 487–491.
Schmiel, D. H., & Miller, V. L. (1999). Microbial Infection, 1, 1103–1112.
Istivan, T. S., & Coloe, P. J. (2006). Microbiology, 152, 1263–1274.
Kohler, G. A., Brenot, A., Stapleton, E. H., Agabian, N., Deva, R., & Nigam, S. (2006). Biochimica et Biophysica Acta, 1761/11, 1391–1399.
D’Arrigo, P., & Servi, S. (1997). Trends in Biotechnology, 15, 90–96.
Guo, Z., Vikbjerg, A. F., & Xu, X. (2005). Biotechnology Advances, 23, 203–259.
Aoki, J., Nagai, Y., Hosono, H., Inoue, K., & Arai, H. (2002). Biochimica et Biophysica Acta, 1582, 26–32.
Snijder, H. J., & Dijkstra, B. W. (2000). Biochimica et Biophysica Acta, 1488, 91–101.
Durieux, M. E., & Lynch, K. R. (1993). Trends in Pharmacological Sciences, 14, 249–254.
Palta, J.P. & Farag, K.M. (1992) U.S. patent 5,126,155.
Six, D. A., & Dennis, E. A. (2000). Biochimica et Biophysica Acta, 1488, 1–19.
Matoba, Y., & Sugiyama, M. (2003). Protein, 51, 453–469.
Winter, B. H., Titze, K., & Marschner, V. (1998). European Journal of Lipid Science and Technology, 100, 152–156.
Chen, S., Wright, L. C., Santangelo, R. T., Muller, M., Moran, V. R., Kuchel, P. W., et al. (1997). Infection and Immunity, 65, 405–411.
Katan, M. (1998). Biochimica et Biophysica Acta, 1436, 5–17.
Heinz, D. W., Essen, L. O., & Williams, R. L. (1998). Journal of Molecular Biology, 275, 635–650.
Feng, J., Wehbi, H., & Roberts, M. F. (2002). The Journal of Biological Chemistry, 277/22, 19867–19875.
Liscovitch, M., Czamy, M., Fiucci, G., & Tang, X. (2000). The Biochemical Journal, 345, 401–415.
McDermott, M., Wakelam, M. J., & Morris, M. (2004). Biochemistry and Cell Biology, 82, 225–253.
Waite, M. (1999). Biochimica et Biophysica Acta, 1439, 187–197.
Ulbrich-Hofmann, R. (2003). European Journal of Lipid Science and Technology, 105, 305–308.
Hatanaka, T., Negishi, T., Kubota-Akizawa, M., & Hagishita, T. (2002). Biochimica et Biophysica Acta, 1598, 156–164.
Hatanaka, T., Negishi, T., Kubota-Akizawa, M., & Hagishita, T. (2002). Enzyme Microbial Technology, 31, 233–241.
Singer, W. D., Brown, H. A., Jiang, X., & Sternweis, P. C. (1996). The Journal of Biological Chemistry, 271/8, 4504–4510.
Iyer, S. S., & Kusher, D. J. (1999). The Journal of Biological Chemistry, 274/4, 2350–2359.
Reetz, M. T. (2001). Angewandte Chemie International Edition England, 40, 284–310.
Tseng, T. C., & Bateman, D. F. (1969). Phytopathology, 59, 359–363.
Lane, T., & Garcia, J. R. (1991). Mycoses, 34, 105–132.
Iwasaki, Y., Nishikawa, S., Tsuneda, M., Takahashi, T., & Yamane, T. (2004). Analytical Biochemistry, 329, 157–159.
Lobo De Araujo, A., & Radvanyi, F. (1987). Toxicon, 25, 1181–1188.
Cox, G. M., McDade, H. C., Chen, S. C. A., Tucker, S. C., Gottredsson, M., Wright, L. C., et al. (2001). Molecular Microbiology, 39, 166–175.
Eibl, H., & Lands, W. E. (1969). Annual Review of Biochemistry, 30, 51–57.
Cho, W., & Kezdy, J. (1991). In E. Dennis (Ed.), Methods in enzymology (Vol. 197, pp. 75–79). London: Academic.
Jiménez, M., Cabanes, J., Gandia-Herrero, F., Escribano, J., Garcia-Carmona, F., & Perez-Gilabert, M. (2003). Analytical Biochemistry, 319, 131–137.
Durban, M. A., & Bornscheuer, U. T. (2007). European Journal of Lipid Science and Technology, 109, 469–473.
Feng, L., Manabe, K., Shope, J. C., Widmer, S., DeWald, D. B., & Prestwich, G. D. (2002). Chemistry & Biology, 9, 795–803.
Hendrickson, S. H. (1994). Analytical Biochemistry, 219, 1–8.
Liu, Y., Ogawa, K., & Schanze, K. S. (2008). Analytical Chemistry, 80, 150–158.
Cho, K. W., Mo, S. J., Lee, H. S., Rho, J. R., & Shin, J. (2000). Journal of Microbiology, 38, 150–155.
Reynolds, L. J., Washbuen, W. N., Deems, R. A., & Dennis, E. A. (1991). Methods in Enzymology, 197, 3–23.
Petersen, G., Chapman, K. D., & Hansen, H. S. (2000). Journal of Lipid Research, 41, 1532–1537.
Balet, C., Clingman, K. A., & Hajdu, J. (1988). Biochemistry Biophysics Research Communication, 150, 561–567.
D’Arrigo, P., Piergianni, V., Scarcelli, D., & Servi, S. (1995). Analytica Chimica Acta, 304, 249–254.
Fliegera, A., Gong, S., Faigle, M., & Neumeister, B. (2000). Enzyme and Microbial Technology, 26, 451–458.
Maity, G., & Bhattacharyya, D. (2005). Current Science, 89, 1004–1008.
Frank, D., & Gunstone, S. (1999). Journal of Science Food Agriculture, 79/12, 1535–1549.
Hosokawa, M., Ito, M., & Takahashi, K. (1998). Biotechnology Techniques, 12, 583–586.
Aura, A. M., Forsell, P., Mustranta, A., & Pautanen, K. (1995). Journal of the American Oil Chemists’ Society, 72, 1375–1379.
Hosokawa, M., Takashaki, K., Kikuchi, Y., & Hatanio, M. (1995). Journal of the American Oil Chemists’ Society, 72, 1287–1291.
Maruha, H. (1996) Pat J P 08168390.
Suzuki, S., Yamatoya, H., Sakai, M., Kataoka, A., & Kudo, S. (2001). The Journal of Nutrition, 131, 2951–2956.
Ulbrich-Hofmann, R., Lerechner, A., Oblozinsky, M., & Bezzkova, L. (2005). Biotechnology Letters, 27, 535–544.
Kyowa, H. (1992) Pat. EP493045.
Ansell, G. B., & Hawthorne, J. N. (1964). In G. B. Ansell & J. N. Hawthorne (Eds.), Phospholipase (pp. 152–174). Amsterdam: Elsevier.
Aalrust, E., Beyerw, S., Ottofrickenstein, H., Penk, G., Plainer, H. & Reiner, R. (1993) United States Patent 5264367.
Clausen, K. (2001). European Journal of Lipid Science and Technology, 103, 333–340.
Kosugi, K. (1999) Pat J.P. 11228986
Gramatikova, S., Hazlewood, G., Lam, D., Borton, N.R., Sturgis, B.G., Robertson, D.E., Li, J., Kreps, J.A., Fielding, R., Brown, R.C., Vasavada, A., Tan, X., Badillo, A., Van Hock, W.P., Janssen, G., Isaac, C. & Burk, M.J. (2005) USA, PCT, International patent Application WO2005 5086900.
Dahlke, K., Buchold, H., Munch, E. M., & Paulitz, B. (1995). Inform, 6, 1284–1291.
Buchold, H. (1993). European Journal of Lipid Science and Technology, 95, 300–304.
Sproessler, B., Loeffler, F., Ottefrickenstein, H. & Plainer, H. (1997) Pat. D E 19527274.
Dutilh, A. C., & Groger, W. (1981). Journal of the Science of Food and Agriculture, 32, 451–458.
Yoshinori, M. (1997). Journal of Agriculture and Food Chemistry, 45, 4558–4563.
Clausen, I.G., Patkar, S.A., Borch, K., Halkier, T., Barfoed, M., Clausen, K., Fuglsang, C.C. & Dybdal, L. (1998) Novoenzyme A/S Denmark. PCT International Patent application WO1998/26057.
Albermann, K., Kemmner, W., Kimpel, E., Maier. D., Spreafico. F., Stock. A., Wagner, C., Boer D.L., Meima, R.B. & Bernhard, R.M. (2003) DSM, The Netherlands. PTC International Patent Application WO2003097825.
Beudeker, R.F. & Kies, A.K. (1997) Pat EP 743017.
Yakult, H. (1991) Pat. WQO9011823.
Yoon, S. H., Lee, C. H., Kim, D. Y., Kim, J. W., & Park, K. H. (1994). Journal of Food Science, 59, 490–493.
Farkye, N. Y. (2004). International Journal of Dairy Technology, 57(2–3), 91–98.
Nielsen, E. W. (2004). In Y. H. Hui, L. Meunier-Goddik, A. Solvejg Hansen, J. Josephsen, W. K. Nip, P. S. Stanfield, & F. Toldra (Eds.), Hand book of food and beverage fermentation technology (pp. 221–239). New York: Marcel Dekker.
Nielsen, P.M. (2002) US Pat. No. 6,399,121.
Lilbaek, H. M., Broe, M. L., Hoier, E., Fatum, T. M., Ipsen, R., & Sorensen, N. K. (2006). Journal of Dairy Science, 89/11, 4114–4125.
Shimizu, T., Ohto, T., & Kita, Y. (2006). IUBMB Life, 58, 238–333.
Songer, J. G. (1997). Trends in Microbiology, 156, 156–161.
Titball, R. W. (1993). Microbiological Reviews, 57, 347–366.
Awad, M. M., Bryant, A. E., Stevens, D. L., & Rood, J. I. (1995). Molecular Microbiology, 15, 191–202.
Srevens, D. L., Tweten, R., Awad, M. M., Road, J. L., & Bryant, A. E. (1997). Journal of Infectious Disease, 176, 189–195.
Williamson, E. D., & Titball, R. W. (1993). Vaccine, 11, 1253–1258.
Leimeister-Wachter, M., Domann, E., & Chakraborty, T. (1991). Molecular Microbiology, 5, 361–366.
Gilmore, M. S., Cruz-Rodz, A. L., Leimeister Watcher, M., Kreft, J., & Goebel, W. A. (1989). Journal of Bacteriology, 171, 744–753.
Wazny, T. K., Mummaw, N., & Styrt, B. (1990). European Journal of Clinical Microbiology & Infectious Diseases, 9/11, 830–832.
Yozwiak, M. L., & Songer, J. G. (1993). American Journal Veterinary Research, 54, 392–397.
Hanel, H., Kirch, R., Schmidts, H. L., & Kottmann, H. (1995). Mycoses, 38, 251–264.
Song, J. W., & Rhee, J. S. (2000). Applied and Environmental Microbiology, 66/3, 890–894.
Erb-Downward, J. R., & Huffnagle, G. B. (2006). Future Microbiology, 1, 219–227.
Noverr, M. C., Phare, S. M., Toews, G. B., Coffey, M. J., & Huffnagle, G. B. (2001). Infection and Immunity, 69, 2957–2963.
Noverr, M. C., Toews, G. B., & Huffnagle, G. B. (2002). Infection and Immunity, 70, 400–402.
Noverr, M. C., Erb-Downward, J. R., & Huffnagle, G. B. (2003). Clinical Microbiology Reviews, 16, 517–533.
Noverr, M. C., Cox, G. M., Perfect, J. R., & Huffnagle, G. B. (2003). Infection and Immunity, 71, 1538–1547.
Macphee, C. H., Nelson, J. J., & Zalewski, A. (2005). Current Opinion in Lipidology, 16, 442–446.
Meyer, M. C., Rastogi, P., Beckett, C. S., & McHowat, J. (2005). Current Pharmaceutical Design, 11, 1301–1312.
Kammoun, M., Koubaa, I., Ali, R. J., Gargouri, Y., Damak, M., & Bezzine, S. (2010). Applied Biochemistry and Biotechnology, 162, 662–670.
Souza, D. L., Rodrrgues-Piiho, E., Souza, Q. L., Pereira, J. O., Calgarotto, A. K., Maso, V., et al. (2007). Toxicon, 51, 240–250.
Acknowledgment
Funding to LR for this work by Council of Scientific and Industrial Research, New Delhi, India is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramrakhiani, L., Chand, S. Recent Progress on Phospholipases: Different Sources, Assay Methods, Industrial Potential and Pathogenicity. Appl Biochem Biotechnol 164, 991–1022 (2011). https://doi.org/10.1007/s12010-011-9190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9190-6