Abstract
Purpose of Review
There have been significant advances in the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL) over the past two decades. However, the intention of treatment remains control of the disease and delay of progression rather than a cure which remains largely elusive. Considering that CLL is mostly seen in older patients, there are multiple factors that play a role in the selection of CLL beyond the frontline treatment. Here, we review the concept of relapsed CLL, factors that predispose to relapse, and therapeutic options available to this patient population. We also review investigational therapies and provide a framework for selection of therapies in this setting.
Recent Findings
Targeted therapies with continuous BTK inhibitors (BTKi) or fixed duration venetoclax plus anti-CD20 monoclonal antibody therapy have established superiority over chemoimmunotherapy in relapsed CLL and have become the preferred standard of care treatment. The second-generation more selective BTK inhibitors (acalabrutinib and zanubrutinib) have shown improved safety profile compared to ibrutinib. However, resistance to the covalent BTK inhibitors may emerge and is commonly associated with mutations in BTK or other downstream enzymes. The novel non-covalent BTK inhibitors such as pirtobrutinib (Loxo-305) and nemtabrutinib (ARQ 531) are showing promising activities for relapsed CLL refractory to prior covalent BTKi. Other novel therapies such as chimeric antigen receptor (CAR) T cell therapy have also shown significant activities for relapsed and refractory CLL. Measurable residual disease (MRD) assessment has a growing importance in venetoclax-based limited-duration therapy and there is mounting evidence that MRD negativity improves outcomes. However, it remains to be seen if this will become an established clinically significant endpoint. Further, the optimal sequence of various treatment options remains to be determined.
Summary
Patients with relapsed CLL now have more options for the treatment of the disease. The choice of therapy is best individualized especially in the absence of direct comparisons of targeted therapies, and the coming years will bring more data on the best sequence of use of the therapeutic agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last decade has seen a significant increase in the therapeutic options available to patients with chronic lymphocytic leukemia (CLL). The treatment armamentarium has expanded beyond traditional chemoimmunotherapy (CIT) with the introduction of novel small molecule kinase/pathway inhibitors such as Bruton tyrosine kinase inhibitors (BTKi, including ibrutinib, acalabrutinib, and zanubrutinib), BCL-2 inhibitor (BCL-2i, including venetoclax), and phosphatidylinositol 3-kinase inhibitors (PI3Ki, including idelalisib and duvelisib). These treatments may be fixed-duration (for CIT or venetoclax-based therapy), or continuous indefinitely (for BTKi or PI3Ki). Present-day treatments have significantly improved the progression-free survival (PFS) and overall survival (OS) in patients with CLL requiring therapy.
Although current frontline treatment is highly effective in achieving a remission, CLL remains incurable. The disease will likely relapse and progress requiring further line(s) of therapy.
Relapse is defined as progression of CLL after achieving partial or complete remission for at least 6 months. This is different from refractoriness which represents non-response to therapy, progression while on treatment, or progression within 6 months of completion of a time-limited therapy. In this review, we will be discussing treatments for both relapsed and refractory CLL. Patients with progressive disease does not necessarily require treatment unless they are meeting one of the treatment indications based on the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria for progressive disease and indication for treatment [1].
While the use of approved agents in the frontline setting is well established, the sequence of deploying the available therapeutic agents in the relapsed/refractory setting is still evolving and remains the subject of investigation. Therefore, the selection of therapies for patients with relapsed/refractory CLL requires consideration of several factors including previous therapy, disease characteristics, the patient’s clinical status (including co-morbidities, concurrent medications etc.), access to clinical trials, and cost implications [2]. Here we review the available clinical trial data and propose a treatment algorithm for relapsed/refractory CLL.
Frontline Treatment of CLL
The options for the initial treatment of patients with active CLL have expanded significantly in the last two decades with targeted agents gradually replacing traditional CIT as the new standard of care, which is further accelerated by the onset of the COVID-19 pandemic.
Currently available targeted therapies in the upfront setting include BTK inhibitors as continuous treatment (with or without anti-CD20 monoclonal antibody), and venetoclax combined with anti-CD20 monoclonal antibody (mAb). These agents are highly efficacious and have demonstrated PFS and OS benefits when compared with traditional CITs in multiple large randomized phase III studies [2,3,4,5,6,7,8]. The initial results from the ECOG 1912 clinical trial suggested that there was no PFS difference in patients with IgHV-mutated CLL without del(17p) or TP53 mutation treated with ibrutinib compared to those treated with the CIT regimen, fludarabine-cyclophosphamide-rituximab [9]. However, the 5-year follow-up results from the study, which demonstrated a PFS benefit with ibrutinib in all subgroups, have shown that targeted therapies, when available, are the best initial treatment modality in patients with CLL [10].
The choice of treatment is determined by patient’s preference (including time-limited versus continuous treatment), patient characteristics (including functional status and comorbidities), disease characteristics (standard versus high risk), and costs.
Predictors of Relapse/Prognosis in CLL
Certain unfavorable prognostic factors have been identified in CLL. These include an unmutated IGHV gene; cytogenetic abnormalities such as complex karyotype, del(17p) or TP53 mutation, etc. which have all been demonstrated to be strong predictors of treatment-free interval and overall survival. In particular, del(17p) and/or TP53 mutation portend a shorter treatment-free interval, poor response to standard chemotherapy, and shorter survival [11,12,13,14,15,16,17,18,19]. Importantly, the timing of progression of disease is an important prognostic factor in patients with relapsed CLL. Early progression of disease — defined as within 24 months of frontline therapy — is an established indicator of inferior response rate to subsequent therapy and survival [20]. Several prognostic models have been developed to predict outcomes in patients with CLL. These include the International Prognostic Index for CLL (CLL-IPI), the Integrated Scoring System (ICSS), the more recent prognostic models that predict outcomes in patients treated with targeted therapy [21,22,23].
The setting of relapse is also important as patients who relapse or progress while on continuous therapy typically indicate refractory disease, warranting a switch to another drug class due the development of resistance (mechanisms discussed under each targeted therapy class below). Relapsed disease after finite-limited therapy can be treated with the same regimen provided the duration of response to the initial therapy is adequate.
Relapse of CLL or Richter’s Transformation?
When CLL patients are presenting with rapidly progressive disease, it is important to consider the possibility of Richter transformation (RT), which represents the emergence of an aggressive lymphoma, most commonly diffuse large B cell lymphoma, and less commonly Hodgkin lymphoma [24]. It is important to make a distinction between relapsed CLL and RT as the treatments are quite different and the latter portends a dismal prognosis [25]. The clinical presentation of RT often overlaps with that of relapsed CLL with the exception that the symptomatology of RT is usually more pronounced, resulting in greater morbidity. The findings that often draws attention to the possibility of RT include elevated serum LDH, asymmetrical rapid increase of lymph node size, and hypercalcemia as seen in about eighty-two percent of patients in a retrospective review study [26]. The diagnosis of Richter’s transformation always requires tissue biopsy of the suspected site of transformation, which can be selected based on markedly increased FDG uptake on positron emission tomography (PET) with computed tomography [27]. Notably, the phenomenon of pseudo-Richter transformation has been described by us and others in patients in whom a temporary hold of BTKi resulted in clinical progression (as evidenced by progressive lymphadenopathy, cytopenia, lymphocytosis) with histopathologic evidence of large cell lymphoma but subsequent resolution of clinical and histopathologic changes upon resumption of therapy with the BTKi [28]. Therefore, this pitfall should be recognized.
RT is typically treated with aggressive chemoimmunotherapy although the response tends to be short-lasting, and prognosis is poor. In younger and fit patients, allogeneic stem cell transplant should be considered once response is achieved with CIT as it improves the chance of long-term survival.
Treatment for Relapsed/Refractory CLL
BTK Inhibitors
The B cell receptor signaling pathways are key to the pathogenesis of CLL by the way of antigen-independent cell-autonomous activation, and one of the core proteins involved is the Bruton’s tyrosine kinase (BTK) — effects lead to sustained inhibition of the B cell receptor and promotion of tumor proliferation [29, 30].
BTK inhibitors have revolutionized the treatment of CLL — including in the relapsed setting — since the in approval of the first-in-class agent, ibrutinib, in 2014. Ibrutinib was first studied in relapsed CLL on the phase Ib/II PCYC-1102 study and efficacy was confirmed by a multicenter randomized control trial (RCT), RESONATE comparing single agent ibrutinib to ofatumumab [31, 32]. After 6-years of follow-up, ibrutinib demonstrated superior outcomes with a significantly longer median PFS (44.1 vs. 8.1 months; hazard ratio [HR]: 0.148; 95% confidence interval [CI]: 0.113–0.196; P˂.001) and median OS (HR: 0.639; 95% CI: 0.418–0.975) after the latter parameter was censored for the extensive cross-over which occurred from the ofatumumab arm to the ibrutinib arm upon progression [33]. Importantly, the superiority of ibrutinib was maintained in the patients with high risk molecular/cytogenetic abnormalities such as unmutated IGHV status, TP53 mutation, del(17p), and del(11q) as the median PFS was 44.1 vs. 8.0 months (HR: 0.110; 95% CI: 0.080–0.152) [33].
In the data obtained from long term of patients in the RESONATE study, the most frequently reported grade ≥3 toxicities related to ibrutinib include neutropenia (25%), pneumonia (21%), major hemorrhage (10%), hypertension (9%), thrombocytopenia (10%), anemia (9%), urinary tract infection (7%), diarrhea (7%), and atrial fibrillation (6%) [33]. While, the study authors demonstrated that the prevalence of any ibrutinib-related grade ≥3 adverse event decreased after the first year, it is unclear if this is due to discontinuation of the medication in affected patients. Disease progression and adverse events (particularly atrial fibrillation, infections, hemorrhage, and diarrhea) are the major reasons for discontinuation of ibrutinib in patients with CLL with a discontinuation rate of 72% within 5 years in the follow-up data from the study by Byrd et al. and approximately 50% in a real-world analysis of use of ibrutinib in the USA by Mato et al. [34, 35].
Acalabrutinib is the second-generation, highly selective BTK inhibitor that is approved for use in patients with CLL. The approval was based on a global, multicenter, randomized, open-label, phase III study, ASCEND, which established its efficacy of single acalabrutinib compared with investigator’s choice of stand of care treatment with either rituximab combined bendamustine, or idelalisib in patients with relapsed/refractory CLL [36]. The authors reported a relative reduction in the risk of progression or death was 69% with acalabrutinib monotherapy (HR, 0.31; 95% CI, 0.20 to 0.49; P < .0001) with median PFS not reached with acalabrutinib monotherapy compared with investigator’s choice (16.5 months [95% CI, 14.0 to 17.1 months) with no different across all disease risk groups [36]. Grade ≥3 adverse events were seen in 45% of patients who received single agent acalabrutinib (most commonly neutropenia, anemia, pneumonia, and atrial fibrillation in 16%, 12%, 5%, and 5% of the patients respectively) versus 86% and 43% of patients who received idelalisib-rituximab and bendamustine-rituximab respectively [36]. The discontinuation rate of acalabrutinib on the ASCEND trial is about 16% but it should be noted that data has only been reported for a 22-month follow-up period [36].
As acalabrutinib is more selective for BTK with fewer off-target kinase inhibition, it was speculated that this might translate into less adverse effects compared to ibrutinib. This was supported by the ELEVATE-RR (ACE-CL-006), an open-label, randomized, non-inferiority, phase 3 trial comparing acalabrutinib with ibrutinib in patients with high-risk relapsed/refractory CLL [37]. In this first direct comparison of less vs. more selective BTKi in CLL, the PFS and OS of patients treated with acalabrutinib were non-inferior to that of patients treated with ibrutinib with a median PFS of 38.4 months (HR 1.00; 95% CI 0.79–1.27) in both groups after a median follow-up of 40.9 months, and the median OS not reached in either group (HR 0.82 [95% CI 0.59–1.15]), with recorded deaths reported at 23.5% and 27.5% in the acalabrutinib and ibrutinib arms respectively [37]. Acalabrutinib also appears to be better tolerated with the study reporting a lower discontinuation rate due to adverse events (14.7 versus 21.3%) compared with ibrutinib [37]. Acalabrutinib was associated with fewer adverse events compared with ibrutinib at any grade including hemorrhage, cardiac arrhythmias, and hypertension [37]. The incidence of any-grade diarrhea (34.6 versus 46.0%), arthralgia (15.8 versus 22.8%), atrial fibrillation (9.4 vs. 16.0%), and hypertension (9.4 versus 23.2%) were lower with acalabrutinib) [37]. However, treatment with acalabrutinib was associated with a higher incidence of headache (34.6 versus 20.2%) and cough (28.9 versus 21.3%) [37].
Thus, acalabrutinib has emerged as a suitable alternative to ibrutinib in patients with CLL, including those who are intolerant to the latter. This was affirmed by the multicenter, single-agent, phase II study, ACE-CL-208, which evaluated the use if acalabrutinib in patients with active relapsed/refractory CLL and intolerance to ibrutinib. In the sixty patients treated with acalabrutinib, overall response rate (ORR) was 73% with 5% of the patients achieving complete remission (CR) [38, 39•]. While the median PFS and OS were not reached at the median follow-up of 35 months, their 24-month estimates were reported as 72% and 81% respectively [39•]. The reported discontinuation rates for disease progression and adverse events were 23% and 17% respectively [39•]. Reported adverse events were similar to those seen in other studies. Eighteen percent of the patients treated with acalabrutinib following intolerance to ibrutinib had recurrence of the same AE experienced while on ibrutinib with diarrhea and bleeding being the most common recurrent AEs. However, only one patient had acalabrutinib discontinued for the same AE, diarrhea albeit lower grade, which had necessitated the discontinuation of ibrutinib [39•]. In a study including 33 patients with ibrutinib intolerance, Awan et al. treated CLL with single agent acalabrutinib and demonstrated that 72 % of the AEs reported with ibrutinib did not recur with acalabrutinib while 24% recurred at the same or lower grade with only 3% of AEs recurring at a higher grade [38].
Zanubrutinib is another highly selective irreversible BTK inhibitor, which is currently in advanced stages of clinical trials. Its efficacy and safety were first demonstrated by an international, open-label, multicenter phase I/II study evaluated the use of zanubrutinib in 122 patients with BTK inhibitor-naïve CLL — 82% had received prior therapy for CLL [40]. The overall response rate was 97% with a composite complete response rate of 14% after a medial follow-up of 25.1 months [40, 41]. While the median PFS was not reached, it was estimated at 97% and 89% at 12 and 24 months respectively [41]. In patients with del(17p), there was no major difference in ORR (94 vs. 97%) but del(17p), but the complete response rate was 6% while PFS rate was 75% at 24 months, consistent with preservation of efficacy in high-risk disease [41]. The most common grade ≥3 AEs in ≥5% of patients were neutropenia (14%), pneumonia (6%), and anemia (6%) but contusion (46%), upper respiratory tract infection (39%), diarrhea (30%), cough (28%), and headache (23%) were the most common any-grade adverse events. Notably, the incidence of atrial fibrillation was 3% with grade ≥3 seen in only 2 of the 122 patients [41]. The overall treatment discontinuation rate was 17% with 13 of the 122 (11%) patients discontinuing zanubrutinib due to disease progression while discontinuation due to adverse events was only reported in only 4 patients (3%) [41]. Xu et al. conducted a single-arm, phase 2 study to evaluate the efficacy and safety of zanubrutinib in 91 Chinese patients with relapsed/refractory CLL. The reported ORR was 84.6% with 3% achieving complete response after a median follow-up of 15.1 months [42]. The 12-month event-free rate and overall survival rate were estimated at 92.9% and 96% respectively [42]. Cytopenias (neutropenia, 44%; thrombocytopenia, 15.4%; anemia, 8.8%) and infections (pneumonia, 13.2%; upper respiratory infection, 9.9%) were the most frequently seen grade ≥ 3 adverse events. No atrial fibrillation or flutter was observed. Adverse events were responsible for the discontinuation of zanubrutinib in 9% of patients [42].
These findings formed the basis of the global, randomized, phase III trial, ALPINE, which compared zanubrutinib head-to-head with ibrutinib in patients with relapsed/refractory CLL. Published data demonstrated that zanubrutinib is superior to ibrutinib in terms of efficacy and safety. The rate of adverse events leading to discontinuation of the BTK inhibitor was lower for zanubrutinib compared with ibrutinib (16.2 vs. 22.8%). Atrial fibrillation/flutter, a key concern with ibrutinib, was also significantly lower with zanubrutinib vs ibrutinib (5.2 vs. 13.3%); there were fewer discontinuations due to cardiac disorders [43•]. While the rate of neutropenia was higher with zanubrutinib (29.3 vs. 24.4%), grade ≥3 infections were seen less frequently with zanubrutinib (26.5 vs. 28.1%) [43•]. At 24 months, the reported ORR was significantly higher with zanubrutinib versus ibrutinib (83.5 vs. 74.2%) and this superiority was sustained across all risk groups [43•]. Interesting, the zanubrutinib arm had an improved PFS versus ibrutinib, a finding that was not present in the aforementioned acalabrutinib study. Zanubrutinib had a higher 24-month PFS (78.4 vs. 65.9%; hazard ratio for disease progression or death, 0.65; 95% confidence interval 0.49 to 0.86; P = 0.002); this benefit was maintained across major subgroups including among patients with del(17p) and/or TP53 mutation (72.6 vs. 54.6%; HR for disease progression or death, 0.53; 95% CI, 0.31 to 0.88) [43•]. Fewer deaths were also seen in the zanubrutinib arm (HR for death was 0.76, 95% CI, 0.51 to 1.11) [43•]. These results have led to regulatory approval for the use of zanubrutinib in R/R CLL.
Also, the studies of zanubrutinib have allowed the use of anticoagulant and antiplatelet medications and its absorption has not been shown to be affected by gastric acid-reducing medications such as proton pump inhibitors [41].
Considering that BTK inhibitors are used continuously, drug resistance is inevitable with consequence loss of response or progression of disease. While the mechanisms of resistance to BTK inhibitors remain a subject of investigation, the impaired binding of the drug to its target protein caused by acquired mutations has been described as the most common basis of resistance to BTK inhibitors. Secondary BTK mutations involving most commonly Cys481 and rarely Leu528Trp, or mutations in phospholipase C-γ2 have been identified in patients with resistance to covalent BTK inhibitors, i.e., ibrutinib, acalabrutinib, and zanubrutinib. In one institutional study of several clinical trials treatment-naïve and relapsed/refractory CLL treated with ibrutinib, these acquired mutations were identified in 85% of patients suffered progression of disease on the BTKi [44]. Another study found the BTK Cys481 mutation in 30% of patients with relapsed/refractory disease who developed Richter transformation on ibrutinib [45]. The BTK Cys481 mutation, particularly Cys481Ser, directly alters the covalent BTKi binding site whereas the PLC-γ2 mutations alters the downstream signaling [44, 46,47,48].
BCL-2 Inhibitor
The process of programmed cell death is majorly regulated by pro-apoptotic proteins in the B cell CLL/lymphoma 2 (BCL-2) family [49]. Venetoclax (formerly known as ABT-199, a re-engineered drug from navitoclax) is the first and only inhibitor of the anti-apoptotic protein BCL-2-mediated anti-apoptotic intrinsic cellular apoptosis pathway in clinical use [49]. A highly potent oral agent, venetoclax, selectively inhibits BCL-2 and is approved for use in patients with CLL. The initial approval for venetoclax in for CLL was for patients with relapsed disease with del(17p) [50]. The updated results from this phase II, open-label clinical trial with venetoclax monotherapy showed an ORR of 77%, with 20% of the patients achieving CR after a median follow-up of 26.1 months [50]. It was estimated from the intention-to-treat analysis that 30% of the patients achieved MRD with a cutoff of 10-4 cells in the blood while the 24-month estimated PFS was 54% [51]. Cytopenia were the most frequently reported grade ≥3 adverse events, with neutropenia (40%) being the most common reason for dose adjustments and any-grade infection rate of 81% (25% of patients had grade ≥3 infection) [51].
Venetoclax has also been studied and approved in fixed-duration regimens with anti-CD20 monoclonal antibodies. The first of such regimens to be approved was rituximab-venetoclax for relapsed /refractory CLL. The efficacy of combination rituximab-venetoclax in the relapsed/refractory setting was first demonstrated by the phase 1b M13-365 which included 49 patients and showed an ORR of 86%, with 51% of patients achieving a CR [52]. The response was also durable with two-year estimates for the PFS and sustained response of 82% and 89% respectively [52]. The results of long-term follow-up also established the non-inferiority of discontinuation of venetoclax after achieving an undetectable measurable residual disease status versus continuous therapy [53••]. The approval for rituximab-venetoclax was based on the results of a randomized, open-label, phase 3 trial, MURANO, in which 389 patients were assigned to receive either venetoclax-rituximab (venetoclax for up to 2 years and rituximab for the first 6 months) or bendamustine-rituximab (bendamustine and rituximab for 6 months) [54]. The most recently updated results from the MURANO study after a 5-year follow-up period established the superiority of venetoclax-rituximab with a significantly higher 5-year PFS (53.6 versus 17.0 months, hazard ratio [HR], 0.19; 95% CI, 0.15–0.26; p<0.0001) rate and OS (82.1 versus 62.2%. HR, 0.40; 95% CI: 0.26–0.62; p<0.0001) rates [55]. There was an 100% response rate with venetoclax-rituximab (10 of 10 patients) who had been previously treated with ibrutinib although refractoriness to BTK inhibitor and/or PI3K inhibitor therapy, exposure with ≥4 lines of therapy, and lymphadenopathy >5 cm were associated with lower complete response rate and a shorter duration of response [56, 57•]. While the presence of del(17p) — with or without TP53 mutation — and NOTCH1 mutation did not negatively impact the probability of response, they appeared to confer a shorter duration of response [58, 59]. Patients who achieved a negative measurable residual disease (MRD) status at the end of combination therapy (i.e., 6 months) had a superior PFS compared with those who did not at the end of the period, suggesting the importance of MRD negativity as a beneficial clinical endpoint [56, 58]. Genomic complexity conferred lower MRD negativity and PFS rates. BIRC3 and BRAF mutations were associated with lower MRD negativity rates at 6 months while TP53, NOTCH1, XPO1, and BRAF mutations were associated with lower MRD negativity rates at the end of therapy (i.e., 2 years) [58]. Grade ≥3 adverse events were seen more frequently in patients who received venetoclax-rituximab (82 versus 70%) with the most common being neutropenia [54]. While patients who received venetoclax-rituximab had more grade ≥3 neutropenia (57.7 vs. 38.8%), the incidence of grade ≥3 febrile neutropenia and infection was higher in patients treated with bendamustine-rituximab [54]. Tumor lysis syndrome (TLS) is a feared complication of treatment of venetoclax and the rate of grade ≥3 TLS in was higher in the venetoclax-rituximab arm (3.1 versus 1.1%) [54]. With treatment now commonly initiated in the hospital, a ramp-up dosing schedule (over 5 weeks) and standard TLS prophylaxis (with fluids and xanthine oxidase inhibitors) have effective in lowering the risk of TLS especially in patients with bulky disease (defined as lymphadenopathy ≥5 cm with an absolute lymphocyte count of ≥25 × 109/L or lymphadenopathy ≥10 cm alone) [2].
The above data is evidence that time-limited therapy with venetoclax is effective — especially in combination regimens — and safe in patients with relapsed CLL. The rate of disease progression at 24 months after discontinuation of venetoclax is estimated at 32% with a higher incidence in patients with high-risk disease especially those harboring TP53 mutation [59]. For patients who achieved response to fixed duration venetoclax-based therapy and later develop disease relapse, re-treatment with venetoclax-based therapy might be potentially feasible as demonstrated by the M13-365 clinical trial [52]. Thompson et al. recently published their finding of the efficacity of retreatment of R/R CLL with venetoclax-based regimens [57•]. Preliminary results of a pooled analysis presented by Cramer et al. at the 2022 European Hematology Association meeting also suggest that patients who suffer a relapse after fixed duration venetoclax-based regimen can be successfully re-treated with venetoclax with significant rates of regaining MRD negativity [60]. Prospective studies are ongoing to further investigate venetoclax re-treatment strategy.
Relapsed CLL may become refractory to venetoclax-based therapy and the mechanism of venetoclax resistance is poorly understood. There is data suggesting that an acquired point mutation in BCL-2, Gly101Val, results in impaired interaction between venetoclax and its binding site on BCL-2, leading to resistance [61]. However, this is not the universal mechanism of resistance to venetoclax as it has only been identified in some of the patients, suggesting alternative mechanisms of resistance, including mutually exclusive or co-existing non- Gly101Val BCL-2 mutations, upregulation of other anti-apoptotic proteins such as MCL-1 and BCL-xL, and reprogramming of mitochondrial oxidative processes via the ID3, AMPK, and PKA pathways in this patient population [62, 63].
Phosphatidylinositol 3-Kinase δ Inhibitors
The delta isoform of phosphatidylinositol 3-kinase is essential in the functioning of the B cell–receptor signaling pathway which is important in the pathogenesis of CLL [64, 65]. Abundantly expressed on the surface of lymphoid cells, phosphatidylinositol 3-kinase δ is the most vital isoform of phosphatidylinositol 3-kinase δ (PI3K) in the most important isoform of development of CLL and plays a key role in key cellular functions such as survival, metabolism, migration, and proliferation, which are promote survival [65,66,67].
Idelalisib, previously known as CAL-101 and GS-1101, is the first PI3K inhibitor approved for use in relapsed/refractory CLL after clinically activity and safety were demonstrated, as single agent and in combination regimens, in phase I trials [68, 69]. The approval was based on an international, multicenter, randomized, double-blind, placebo-controlled phase III trial which compared a combination regimen of idelalisib-rituximab with rituximab-placebo in 220 patients with relapsed/refractory CLL [70]. The primary end point, PFS, was reported as 5.5 months in the rituximab-placebo arm but not reached in patients treated with idelalisib-rituximab (HR: 0.15; P<0.001). The OS at 12 months was higher in the idelalisib-rituximab arm (92 versus 80%; HR: 0.28; P=0.02). The ORR was also higher in patients treated with idelalisib ((81 versus 13%; odds ratio: 29.92; P<0.001). The presence of high-risk features, such as complex karyotype, del(17p), TP53 mutation and/or del(11q) did not have significant effect on OS in the arm with idelalisib-rituximab [71]. Forty and thirty-five percent of patients in the idelalisib-rituximab and rituximab-placebo arms respectively experienced at least one serious adverse event with the most frequent being pneumonia, pyrexia, and febrile neutropenia [70].
Duvelisib, the second PI3K inhibitor to be approved in patients with relapsed/refractory CLL who have received at least two prior lines of therapy, is an oral dual PI3K δ and γ inhibitor. The approval was based on the results of the global phase III randomized trial, DUO, which compared monotherapy with duvelisib with ofatumumab, with demonstration of significant PFS benefit in the duvelusib arm (13.3 months versus 9.9 months), and this was maintained in patients with progressive disease while on the ofatumumab arm [72].
Toxicity (especially immune-mediated hepatitis and colitis) is a major concern with the first generation PI3K inhibitors and has limited their use to beyond the second line of therapy [73, 74].
A second-generation PI3K inhibitor, umbralisib, has been demonstrated to have clinical efficacy but with a better toxicity profile compared with the first-generation PI3K inhibitors. There were ongoing clinical trials evaluating its combination with other targeted therapies [75, 76]. However, the approval application to the Food and Drug Administration (FDA) for the combination of umbralisib with the novel anti-CD20 monoclonal antibody, ublituximab, was voluntarily withdrawn by the manufacturer in April, 2022 after there was demonstration of survival benefit in the control arm (obinutuzumab-chlorambucil) despite initially promising data[77].
Recently, the first-generation PI3K inhibitors have seen their safety called into doubt with recent reviews of additional data by the FDA raising safety concerns for their use in indolent non-Hodgkin lymphoma including R/R CLL/SLL. The withdrawals (voluntary and involuntary) have been based on results of clinical trials (including several halted trials due to increased risk of death, e.g., NCT01732913) which showed an increased risk of harm (including fatal infections) related to the use idelalisib and duvelisib in studies including patients with CLL and other R/R non-Hodgkin lymphoma [72].
Chemoimmunotherapy
The use of traditional chemotherapy agents such as alkylating agents (such as bendamustine and chlorambucil), and purine analogs (such as fludarabine and pentostatin) in combination with the anti-CD20 monoclonal antibodies was previously the mainstay of the treatment of CLL but has significant decreased since the introduction of pathway inhibitors, especially with the COVID-19 pandemic during which there has been increased concern for increased risk of infections and therapy-related mortality. Prior to the introduction of pathway inhibitors, the combination of anti-CD20 monoclonal antibodies with chemotherapy was the mainstay of treatment of relapsed/refractory immunotherapy. Combination regimens using traditional chemotherapy agents such as alkylating agents (such as bendamustine and chlorambucil), and purine analogs (such as fludarabine and pentostatin) have been shown to provide significant PFS benefit and is viable options in patients who cannot receive or have access to pathway inhibitors [78,79,80].
While there are no prospective studies that have evaluated the use of conventional chemoimmunotherapy (CIT) after frontline targeted therapy, it appears that CIT is inferior to targeted therapy in the relapsed/refractory setting. A retrospective study found that the use of a kinase inhibitor (KI) or venetoclax after initial treated with targeted therapy led to better outcomes compared with CIT with ORRs reported as 58.5%, 73.6%, and 49.9% for KI-based therapy, venetoclax, and CIT respectively with corresponding CR rates of 4.1%, 31.5%, and 2.1% respectively [81]. In patients whose disease (non-high risk, i.e., IGHV-mutated and without 17p deletion or TP53 mutation) relapse after a long period of remission following initial treatment with chemoimmunotherapy, re-treatment with chemoimmunotherapy is an alternative to pathway inhibitors albeit less preferred.
Hemopoietic Stem Cell Transplantation
Hemopoietic stem cell transplantation (HSCT), usually allogeneic for the purpose of durable graft-versus-leukemia effect, is an option for patients for relapsed CLL. Considering that most patients with CLL are older than 70 years and there are now more effective options for treatment of the disease, HSCT is not widely used due to the intensity of the treatment and the associated risk of morbidity and mortality. The data for the use of HSCT is largely retrospective with a few prospective trials but no randomized study comparing it other therapies [82,83,84].
The timing of HSCT in the sequence of treatment of relapsed disease remains unknown. The European Research Initiative on CLL and European Society for Blood and Marrow Transplantation have proposed a framework that suggests that the patients suitable for HSCT are those with relapsed high-risk CLL resistant to both chemoimmunotherapy and targeted therapies (BTK inhibitor and/or BCL2 inhibitor) [85]. Preferably, the use of HSCT should be reserved for younger patients, with progression after targeted therapies, in the setting of a clinical trial.
Promising Novel Experimental Therapies
Non-covalent BTK Inhibitors
Due to eventual emergence of resistance to covalent BTK inhibitors, there have been efforts at developing noncovalent BTK inhibitors which exert their effect on BTK by bring to sites other than C481 site targeted by covalent BTK inhibitors. Two such agents, pirtobrutinib (formerly LOXO-305) and nemtabrutinib (formerly ARQ-531), have been demonstrated to be safe and efficacious in early phase clinical trials. In the BRUIN study, a multicenter, open-label, phase I/II study to evaluate pirtobrutinib in R/R B cell malignancies including CLL/SLL and previous exposure to BTK inhibitors, the ORR was 62% [86••]. Resistance to covalent BTK inhibitor does not appear to make a difference as the ORR was similar in patients with no BTK resistance and those who had developed resistance to BTK inhibitors (66% in the patients with wild-type BTK and 71% in those with C481-mutant BTK) [86••]. The most common AE were fatigue (20%) and diarrhea (17%) while neutropenia was the most common grade ≥3 AE. There was no report of grade ≥3 atrial fibrillation/flutter [86••].
Nemtabrutinib, another novel non-covalent BTK inhibitor, has shown promise in both wild-type and C481-mutated R/R CLL with publication and recently presented conference abstract being awaited [87••].
While these non-covalent BTK inhibitors have the potential to change the landscape of treatment of CLL especially in the setting of BTK resistance, resistance to this subclass has been described, namely via BTK domain mutations (including A428D, L528W, M437R, T474I, and V416L) and mutation involving a downstream substrate of BTK, PLCγ2 [88•].
CAR T Cell Therapy
Chimeric antigen receptor (CAR) T cell therapy has revolutionized the care of patients with B cell malignancies but remains an investigational therapy in CLL.
Porter et al. reported the treatment of three patients with relapsed/refractory CLL with a CD19 CAR T cells with demonstrable tumor response with one patient’s response lasting greater than 10 months [89].
Subsequently, there have been less stellar results from large prospective trials of CAR T cell therapy in patients with relapsed CLL [90,91,92,93,94]. Long-term results from a randomized dose optimization study of CD19 CAR-T cells demonstrated ORR and CR rate of 44% and 28% respectively with a median OS of 64 months (not reached in patients with CR) and PFS of 40.2 months in patients who achieved a CR (1 month in those without a CR) [94]. Results from the recently published phase 1 TRANSCEND CLL 004 trial evaluating the use of lisocabtagene maraleucel, an autologous CD19-directed CAR T cell, in patients with relapsed/refractory CLL who had received ≥2 lines of therapy showed that 82% and 45% of the evaluable twenty-two patients achieved overall and complete responses, completely [95•].
Adverse events associated with CAR T cell therapy include cytokine release syndrome, neurotoxicity, cytopenia, and hypogammaglobulinemia [95•]. A limitation to the use of autologous CAR T cell therapy is timely access especially with the logistic and timeline issues associated with the collection and production of the cells.
Selection of Treatment Options
Patients with active relapsed CLL following frontline therapy have several options for further treatment of the disease. Factors that influence the selection of therapeutic regimen in the relapsed setting include the following:
-
Patient’s functional status and comorbidities
-
Patient’s preferences (including duration of therapy)
-
Disease characteristics and risk profile
-
Type of frontline therapy
-
Response to frontline therapy
-
Tolerance to frontline therapy
-
Access to targeted therapies
-
Availability and eligibility for a clinical trial
Sequence of Therapies in Relapsed CLL
There is no established sequence of therapy in relapsed CLL and the choice of the next line of therapy depends on factors described above.
Besides patient and disease characteristics, the duration of response to prior therapy is important in the selection of the next line of therapy. Early relapse is generally considered to be disease progression within 2–3 years of initial FCR, 1 year of other chemoimmunotherapeutic regimens, and 5–6 years of initial targeted therapy [2].
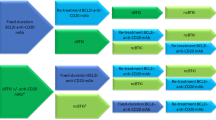
In patients who progress on a BTK inhibitor, a switch to a venetoclax-based therapy is appropriate. Considering that venetoclax-based regimens are time-limited, retreatment with the same regimen can be done in patients with late first relapse. Otherwise, a BTK inhibitor is appropriate [96].
In patients previously treated with chemoimmunotherapy — a modality which is no longer recommended in the frontline setting, regardless of IgHV and/or del(17p)/TP53 status, and should be reserved for patients with no access to targeted therapies — the next line of therapy should be a pathway inhibitor. However, there is no data to support the choice of a BTK inhibitor or venetoclax-based regimen in the relapsed setting as there is no head-to-head comparison of the two classes. A meta-analysis compared treatment of relapsed CLL with ibrutinib or venetoclax and reported no difference in PFS or OS between the two groups [97]. In contrast, a comparative “real world” analysis suggested that treatment venetoclax as first targeted agent provided a longer PFS compared with ibrutinib [98]. Also, there is the suggestion that ibrutinib may offer better outcomes compared with venetoclax-based regimens in patients with del(17p) or TP53 mutation [7, 99].
Therefore, there have been arguments for the use of single agent BTK inhibitors in patients with first relapse of targeted therapy naïve patients with CLL based on the established efficacy, convenience, low concern for tumor lysis syndrome, and avoidance of further suppression of immunotherapy, by the anti-CD20 monoclonal antibody component of venetoclax-based regimens, especially the ongoing COVID-19 pandemic is taken into consideration [100•]. In contrast, venetoclax-based regimens are time-limited, better tolerated especially with a better cardiovascular side effect profile, and offer opportunities for retreatment in patients who suffer a late relapse [101•].
Based on these, below are some general principles that could guide the sequence of therapy [81, 102••]:
-
Patients treated with frontline chemoimmunotherapy should be offered targeted therapy especially if high-risk disease and/or a short duration of response.
-
Patients who progress of BTK inhibitor should be treated with a non-BTK inhibitor-based regimen such as venetoclax in combination with an anti-CD20 monoclonal antibody.
-
Patients with intolerance to Ibrutinib can be treated with another BTK inhibitor (acalabrutinib or Zanubrutinib.
-
The use of PI3K inhibitors should be reserved for patients with progressive disease after ≥2 lines of therapy.
-
The use of allogeneic HSCT can be considered in younger patients (<70 years) with prior exposure to ≥2 targeted therapies and high-risk disease.
-
Enrolment in a CAR T cell therapy clinical trial is reasonable for patients with progressive disease after several lines of targeted therapies.
A general schema for selection of therapies in the relapsed setting is illustrated in Fig. 1.
Measurable Residual Disease Assessment
Major trials of therapeutic agents in relapsed CLL have evaluated the impact of peripheral blood measurable residual disease (also referred to as minimal residual disease) negativity on outcomes with some suggestion of a positive impact of MRD negativity on survival outcomes even in patients treated in chemoimmunotherapy as demonstrated in the CLL111 study which showed that patients who achieved MRD negativity with chlorambucil-obinutuzumab had a significantly longer PFS compared with those who were positive for MRD (median OS not reached versus 19.4 months) [103].
Venetoclax-based regimens have been shown to yield high rates of MRD negativity in patients with relapsed CLL as illustrated by the follow-up results from the MURANO study where 62% patients treated with venetoclax-rituximab achieved MRD negativity after treatment discontinuation and this translated into a significantly longer PFS [56].
However, peripheral blood MRD negativity is often not achieved with ibrutinib monotherapy. Ahn et al. reported an MRD negativity rate of approximately 10% in patients treated with single agent ibrutinib and this did not significantly impact outcomes compared with the control arm [104].
It is important to exercise caution with the application of MRD status as a clinical endpoint in patients with relapsed CLL for several reasons. The sensitivity of current methods of assessing MRD remains suboptimal, and the determination of the best method remains a subject of investigations. Notably, the current MRD assessment in CLL is done using peripheral blood. This represents a major pitfall as CLL is a multifocal disease with sites of disease involvement including lymph nodes, bone marrow, spleen, and liver. Therefore, MRD negativity in the peripheral blood does not necessarily mean same in other disease sights. Also, the biology of the disease — especially cytogenetic and molecular aberrations — exerts significant influence on disease outcomes and could modulate the impact of MRD status [105, 106•].
In clinical practice, the success of ibrutinib monotherapy, despite a low MRD negative rate, suggests that MRD negative may not have the same impact in patients with CLL as seen in patients with other hematologic diseases such as acute lymphoblastic leukemia.
Conclusion
Relapsed CLL can be treated with a variety of options depending on patient, disease, and access factors. The options are efficacious and improve outcomes in this patient population. However, more studies are needed to determine the best sequence of use of the various therapeutic options. Also, better understanding of the mechanisms of resistance to the agents will help guide the development of novel therapies in a bid to improve on the available treatment options. The coming years will also bring more data for various novel combinations of different classes of targeted therapy which hopefully will reduce the incidence of drug resistance and allow for more time-limited treatment options. The jury is still out on use of measurable residual disease in CLL, and it is expected that ongoing and future studies will provide clarity on its clinical benefit.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60. https://doi.org/10.1182/blood-2017-09-806398.
Moreno C. Standard treatment approaches for relapsed/refractory chronic lymphocytic leukemia after frontline chemoimmunotherapy. Hematol Am Soc Hematol Educ Program. 2020;2020(1):33–40. https://doi.org/10.1182/hematology.2020000086.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–28. https://doi.org/10.1056/NEJMoa1812836.
Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–98. https://doi.org/10.1038/s41375-019-0602-x.
Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–91. https://doi.org/10.1016/S0140-6736(20)30262-2.
Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188–200. https://doi.org/10.1016/S1470-2045(20)30443-5.
Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and Obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380(23):2225–36. https://doi.org/10.1056/NEJMoa1815281.
Small S, Ma S. Frontline treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): Targeted therapy vs. chemoimmunotherapy. Curr Hematol Malig Rep. 2021;16(4):325–35. https://doi.org/10.1007/s11899-021-00637-1.
Shanafelt TD, Wang XV, Kay NE, Hanson CA, O'Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–43. https://doi.org/10.1056/NEJMoa1817073.
Shanafelt TD, Wang XV, Hanson CA, Paietta EM, O'Brien S, Barrientos J, et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140(2):112–20. https://doi.org/10.1182/blood.2021014960.
Zapatka M, Tausch E, Ozturk S, Yosifov DY, Seiffert M, Zenz T, et al. Clonal evolution in chronic lymphocytic leukemia is scant in relapsed but accelerated in refractory cases after chemo(immune) therapy. Haematologica. 2022;107(3):604–14. https://doi.org/10.3324/haematol.2020.265777.
Zenz T, Eichhorst B, Busch R, Denzel T, Habe S, Winkler D, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28(29):4473–9. https://doi.org/10.1200/JCO.2009.27.8762.
Landau DA, Tausch E, Taylor-Weiner AN, Stewart C, Reiter JG, Bahlo J, et al. Mutations driving CLL and their evolution in progression and relapse. Nature. 2015;526(7574):525–30. https://doi.org/10.1038/nature15395.
Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–6. https://doi.org/10.1056/NEJM200012283432602.
Thompson PA, O'Brien SM, Xiao L, Wang X, Burger JA, Jain N, et al. beta2 -microglobulin normalization within 6 months of ibrutinib-based treatment is associated with superior progression-free survival in patients with chronic lymphocytic leukemia. Cancer. 2016;122(4):565–73. https://doi.org/10.1002/cncr.29794.
Ibrahim S, Keating M, Do KA, O'Brien S, Huh YO, Jilani I, et al. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood. 2001;98(1):181–6. https://doi.org/10.1182/blood.v98.1.181.
Orchard JA, Ibbotson RE, Davis Z, Wiestner A, Rosenwald A, Thomas PW, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004;363(9403):105–11. https://doi.org/10.1016/S0140-6736(03)15260-9.
Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–54.
Rossi D, Cerri M, Deambrogi C, Sozzi E, Cresta S, Rasi S, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res. 2009;15(3):995–1004. https://doi.org/10.1158/1078-0432.CCR-08-1630.
Ahn IE, Farber CM, Davids MS, Grinblatt DL, Kay NE, Lamanna N, et al. Early progression of disease as a predictor of survival in chronic lymphocytic leukemia. Blood Adv. 2017;1(25):2433–43. https://doi.org/10.1182/bloodadvances.2017011262.
Gentile M, Shanafelt TD, Mauro FR, Reda G, Rossi D, Laurenti L, et al. Predictive value of the CLL-IPI in CLL patients receiving chemo-immunotherapy as first-line treatment. Eur J Haematol. 2018; https://doi.org/10.1111/ejh.13149.
Ahn IE, Tian X, Ipe D, Cheng M, Albitar M, Tsao LC, et al. Prediction of outcome in patients with chronic lymphocytic leukemia treated with ibrutinib: development and validation of a four-factor prognostic model. J Clin Oncol. 2021;39(6):576–85. https://doi.org/10.1200/JCO.20.00979.
Soumerai JD, Ni A, Darif M, Londhe A, Xing G, Mun Y, et al. Prognostic risk score for patients with relapsed or refractory chronic lymphocytic leukaemia treated with targeted therapies or chemoimmunotherapy: a retrospective, pooled cohort study with external validations. Lancet Haematol. 2019;6(7):e366–e74. https://doi.org/10.1016/S2352-3026(19)30085-7.
Wang Y, Tschautscher MA, Rabe KG, Call TG, Leis JF, Kenderian SS, et al. Clinical characteristics and outcomes of Richter transformation: experience of 204 patients from a single center. Haematologica. 2020;105(3):765–73. https://doi.org/10.3324/haematol.2019.224121.
Abrisqueta P, Delgado J, Alcoceba M, Oliveira AC, Loscertales J, Hernandez-Rivas JA, et al. Clinical outcome and prognostic factors of patients with Richter syndrome: real-world study of the Spanish Chronic Lymphocytic Leukemia Study Group (GELLC). Br J Haematol. 2020;190(6):854–63. https://doi.org/10.1111/bjh.16748.
Tsimberidou AM, O'Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24(15):2343–51. https://doi.org/10.1200/JCO.2005.05.0187.
Michallet AS, Sesques P, Rabe KG, Itti E, Tordot J, Tychyj-Pinel C, et al. An 18F-FDG-PET maximum standardized uptake value > 10 represents a novel valid marker for discerning Richter's Syndrome. Leuk Lymphoma. 2016;57(6):1474–7. https://doi.org/10.3109/10428194.2015.1099643.
Barnea Slonim L, Ma S, Behdad A, Chen Q. Pseudo-Richter transformation of chronic lymphocytic leukaemia/small lymphocytic lymphoma following ibrutinib interruption: a diagnostic pitfall. Br J Haematol. 2020;191(1):e22–e5. https://doi.org/10.1111/bjh.16948.
Duhren-von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489(7415):309–12. https://doi.org/10.1038/nature11309.
Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28(3):649–57. https://doi.org/10.1038/leu.2013.358.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. https://doi.org/10.1056/NEJMoa1215637.
Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–23. https://doi.org/10.1056/NEJMoa1400376.
Munir T, Brown JR, O'Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–63. https://doi.org/10.1002/ajh.25638.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum K, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918–27. https://doi.org/10.1158/1078-0432.CCR-19-2856.
Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–9. https://doi.org/10.3324/haematol.2017.182907.
Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: phase III, Randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–61. https://doi.org/10.1200/JCO.19.03355.
Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52. https://doi.org/10.1200/JCO.21.01210.
Awan FT, Schuh A, Brown JR, Furman RR, Pagel JM, Hillmen P, et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3(9):1553–62. https://doi.org/10.1182/bloodadvances.2018030007.
• Rogers KA, Thompson PA, Allan JN, Coleman M, Sharman JP, Cheson BD, et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica. 2021;106(9):2364–73. https://doi.org/10.3324/haematol.2020.272500. Supports the safe use of acalabrutinib in patients with intolerance to ibrutinib.
Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–9. https://doi.org/10.1182/blood.2019001160.
Cull GSD, Opat S, et al. Treatment with the Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) demonstrates high overall response rate and durable responses in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): updated results from a phase 1/2 trial. Blood. 2019;140(2):112–20. https://doi.org/10.1182/blood.2021014960.
Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, et al. Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol. 2020;13(1):48. https://doi.org/10.1186/s13045-020-00884-4.
• Brown JR, Eichhorst B, Hillmen P, Jurczak W, Kazmierczak M, Lamanna N, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319–32. https://doi.org/10.1056/NEJMoa2211582. Demonstrated non-inferiority and better safety profile of zanubrutinib compared to ibrutinib.
Woyach JA, Ruppert AS, Guinn D, Lehman A, Blachly JS, Lozanski A, et al. BTK(C481S)-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J Clin Oncol. 2017;35(13):1437–43. https://doi.org/10.1200/JCO.2016.70.2282.
Kadri S, Lee J, Fitzpatrick C, Galanina N, Sukhanova M, Venkataraman G, et al. Clonal evolution underlying leukemia progression and Richter transformation in patients with ibrutinib-relapsed CLL. Blood Adv. 2017;1(12):715–27. https://doi.org/10.1182/bloodadvances.2016003632.
Handunnetti SMTC, Nguyen T, et al. BTK Leu528Trp - a Potential secondary resistance mechanism specific for patients with chronic lymphocytic leukemia treated with the next generation BTK inhibitor zanubrutinib. Blood. 2019;134(Supplement_1):170. https://doi.org/10.1182/blood-2019-125488.
Kanagal-Shamanna R, Jain P, Patel KP, Routbort M, Bueso-Ramos C, Alhalouli T, et al. Targeted multigene deep sequencing of Bruton tyrosine kinase inhibitor-resistant chronic lymphocytic leukemia with disease progression and Richter transformation. Cancer. 2019;125(4):559–74. https://doi.org/10.1002/cncr.31831.
Woyach JHY, Rogers K, et al. Resistance to Acalabrutinib in CLL is mediated primarily by BTK mutations. Blood. 2019;134(Supplement_1):504. https://doi.org/10.1182/blood-2019-127674.
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–8. https://doi.org/10.1038/nm.3048.
Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768–78. https://doi.org/10.1016/S1470-2045(16)30019-5.
Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol. 2018;36(19):1973–80. https://doi.org/10.1200/JCO.2017.76.6840.
Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230–40. https://doi.org/10.1016/S1470-2045(17)30012-8.
•• Ma S, Seymour JF, Brander DM, Kipps TJ, Choi MY, Anderson MA, et al. Efficacy of venetoclax plus rituximab for relapsed CLL: 5-year follow-up of continuous or limited- duration therapy. Blood. 2021;138(10):836–46. https://doi.org/10.1182/blood.2020009578. First study to demonstrate the effectiveness of retreatment with venetoclax-based regimen.
Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D'Rozario J, Assouline S, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–20. https://doi.org/10.1056/NEJMoa1713976.
Seymour JF, Kipps TJ, Eichhorst BF, D'Rozario J, Owen CJ, Assouline S, et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood. 2022;140(8):839–50. https://doi.org/10.1182/blood.2021015014.
Kater AP, Seymour JF, Hillmen P, Eichhorst B, Langerak AW, Owen C, et al. Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269–77. https://doi.org/10.1200/JCO.18.01580.
• Thompson MC, Harrup RA, Coombs CC, Roeker LE, Pu JJ, Choi MY, et al. Venetoclax retreatment of patients with chronic lymphocytic leukemia after a previous venetoclax-based regimen. Blood Adv. 2022;6(15):4553–7. https://doi.org/10.1182/bloodadvances.2022007812. Reaffirmed the effectiveness of reteatment with venetoclax-based regimens in R/R CLL.
Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D'Rozario J, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study. J Clin Oncol. 2020;38(34):4042–54. https://doi.org/10.1200/JCO.20.00948.
Roberts AW, Ma S, Kipps TJ, Coutre SE, Davids MS, Eichhorst B, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134(2):111–22. https://doi.org/10.1182/blood.2018882555.
Cramer PFM, Giza A, et al. Retreatment with venetoclax after venetoclax, obinutuzumab +/- ibrutinib: pooled analysis of 13 patients with chronic lymphocytic leukemia (CLL) treated in GCLLSG trials. HemaSphere. 2022;6:539–40. https://doi.org/10.1097/01.HS9.0000845448.71709.3a.
Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ER, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;9(3):342–53. https://doi.org/10.1158/2159-8290.CD-18-1119.
Blombery P, Thompson ER, Nguyen T, Birkinshaw RW, Gong JN, Chen X, et al. Multiple BCL2 mutations cooccurring with Gly101Val emerge in chronic lymphocytic leukemia progression on venetoclax. Blood. 2020;135(10):773–7. https://doi.org/10.1182/blood.2019004205.
Guieze R, Liu VM, Rosebrock D, Jourdain AA, Hernandez-Sanchez M, Martinez Zurita A, et al. Mitochondrial reprogramming underlies resistance to BCL-2 inhibition in lymphoid malignancies. Cancer Cell. 2019;36(4):369–84 e13. https://doi.org/10.1016/j.ccell.2019.08.005.
Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–15. https://doi.org/10.1056/NEJMra041720.
Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–30. https://doi.org/10.1038/nri1056.
Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–88. https://doi.org/10.1182/blood-2010-02-271171.
Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–12. https://doi.org/10.1182/blood-2011-05-352492.
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–7. https://doi.org/10.1182/blood-2013-11-535047.
Barrientos JCFR, Leonard J, et al. Update on a phase I study of the selective PI3Kδ inhibitor idelalisib (GS-1101) in combination with rituximab and/or bendamustine in patients with relapsed or refractory CLL. J Clin Oncol. 2013;31(15_suppl):7017. https://doi.org/10.1200/jco.2013.31.15_suppl.7017.
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. https://doi.org/10.1056/NEJMoa1315226.
Kreuzer KA, Furman RR, Stilgenbauer S, Dubowy RL, Kim Y, Munugalavadla V, et al. The impact of complex karyotype on the overall survival of patients with relapsed chronic lymphocytic leukemia treated with idelalisib plus rituximab. Leukemia. 2020;34(1):296–300. https://doi.org/10.1038/s41375-019-0533-6.
Flinn IW, Hillmen P, Montillo M, Nagy Z, Illes A, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–55. https://doi.org/10.1182/blood-2018-05-850461.
Coutre SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779–86. https://doi.org/10.3109/10428194.2015.1022770.
Davids MS, Kuss BJ, Hillmen P, Montillo M, Moreno C, Essell J, et al. Efficacy and safety of duvelisib following disease progression on ofatumumab in patients with relapsed/refractory CLL or SLL in the DUO crossover extension study. Clin Cancer Res. 2020;26(9):2096–103. https://doi.org/10.1158/1078-0432.CCR-19-3061.
Davids MS, Kim HT, Nicotra A, Savell A, Francoeur K, Hellman JM, et al. Umbralisib in combination with ibrutinib in patients with relapsed or refractory chronic lymphocytic leukaemia or mantle cell lymphoma: a multicentre phase 1-1b study. Lancet Haematol. 2019;6(1):e38–47. https://doi.org/10.1016/S2352-3026(18)30196-0.
Lunning M, Vose J, Nastoupil L, Fowler N, Burger JA, Wierda WG, et al. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2019;134(21):1811–20. https://doi.org/10.1182/blood.2019002118.
Jacobs RJW, Flinn IW, et al. Efficacy and safety of ublituximab in combination with umbralisib (U2) in patients with chronic lymphocytic leukemia (CLL) by treatment status: a sub-analysis of the phase 3 unity-CLL study. Blood. 2021;138(Supplement 1):3726. https://doi.org/10.1182/blood-2021-147460.
Tam CS, O'Brien S, Plunkett W, Wierda W, Ferrajoli A, Wang X, et al. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab). Blood. 2014;124(20):3059–64. https://doi.org/10.1182/blood-2014-06-583765.
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–42. https://doi.org/10.1016/S1470-2045(16)30051-1.
Lamanna N, Kalaycio M, Maslak P, Jurcic JG, Heaney M, Brentjens R, et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2006;24(10):1575–81. https://doi.org/10.1200/JCO.2005.04.3836.
Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients. Ann Oncol. 2017;28(5):1050–6. https://doi.org/10.1093/annonc/mdx031.
Kharfan-Dabaja MA, Pidala J, Kumar A, Terasawa T, Djulbegovic B. Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplant. 2012;47(9):1164–70. https://doi.org/10.1038/bmt.2012.71.
Roeker LE, Dreger P, Brown JR, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020;4(16):3977–89. https://doi.org/10.1182/bloodadvances.2020001956.
Kim HT, Shaughnessy CJ, Rai SC, Reynolds C, Ho VT, Cutler C, et al. Allogeneic hematopoietic cell transplantation after prior targeted therapy for high-risk chronic lymphocytic leukemia. Blood Adv. 2020;4(17):4113–23. https://doi.org/10.1182/bloodadvances.2020002184.
Dreger P, Ghia P, Schetelig J, van Gelder M, Kimby E, Michallet M, et al. High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood. 2018;132(9):892–902. https://doi.org/10.1182/blood-2018-01-826008.
•• Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. https://doi.org/10.1016/S0140-6736(21)00224-5. Reports the development of more potent BTK inhibitors which are able to overcome several known drug-class resistance.
•• Reiff SD, Mantel R, Smith LL, Greene JT, Muhowski EM, Fabian CA, et al. The BTK inhibitor ARQ 531 targets ibrutinib-resistant CLL and richter transformation. Cancer Discov. 2018;8(10):1300–15. https://doi.org/10.1158/2159-8290.CD-17-1409. Reports the development of more potent BTK inhibitors which are able to overcome several known drug-class resistance.
• Wang E, Mi X, Thompson MC, Montoya S, Notti RQ, Afaghani J, et al. Mechanisms of resistance to noncovalent Bruton's tyrosine kinase inhibitors. N Engl J Med. 2022;386(8):735–43. https://doi.org/10.1056/NEJMoa2114110. Elucidated mechanisms of resistance to BTK inhibitors.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. https://doi.org/10.1056/NEJMoa1103849.
Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–73. https://doi.org/10.1182/blood-2013-06-506741.
Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–21. https://doi.org/10.1200/JCO.2015.64.5929.
Koehler P, Schmidt P, Hombach AA, Hallek M, Abken H. Engineered T cells for the adoptive therapy of B-cell chronic lymphocytic leukaemia. Adv Hematol. 2012;2012:595060. https://doi.org/10.1155/2012/595060.
Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010–20. https://doi.org/10.1200/JCO.2017.72.8519.
Frey NV, Gill S, Hexner EO, Schuster S, Nasta S, Loren A, et al. Long-term outcomes from a randomized dose optimization study of chimeric antigen receptor modified T Cells in relapsed chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2862–71. https://doi.org/10.1200/JCO.19.03237.
• Siddiqi T, Soumerai JD, Dorritie KA, Stephens DM, Riedell PA, Arnason J, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. 2022;139(12):1794–806. https://doi.org/10.1182/blood.2021011895. Early phase clinical trial demonstrating the effectiveness of CAR T-cell therapy in R/R CLL.
Mato AR, Roeker LE, Jacobs R, Hill BT, Lamanna N, Brander D, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589–96. https://doi.org/10.1158/1078-0432.CCR-19-3815.
Molica S, Giannarelli D, Mirabelli R, Levato L, Shanafelt TD. The magnitude of improvement in progression-free survival with targeted therapy in relapsed/refractory chronic lymphocytic leukemia based on prognostic risk category: a systematic review and meta-analysis. Leuk Lymphoma. 2019;60(7):1644–9. https://doi.org/10.1080/10428194.2018.1543882.
Eyre TA, Lamanna N, Roeker LE, Ujjani CS, Hill BT, Barr PM, et al. Comparative analysis of targeted novel therapies in relapsed, refractory chronic lymphocytic leukaemia. Haematologica. 2021;106(1):284–7. https://doi.org/10.3324/haematol.2019.241539.
Allan JN, Shanafelt T, Wiestner A, Moreno C, O'Brien SM, Li J, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials. Br J Haematol. 2022;196(4):947–53. https://doi.org/10.1111/bjh.17984.
• Brem EA, O'Brien S. Is a BTKi or BCL2i preferable for first "novel" therapy in CLL? The case for BTKis. Blood Adv. 2022;6(4):1361–4. https://doi.org/10.1182/bloodadvances.2019001204. Provides insights on sequencing of novel therapies in CLL.
• Seymour JF. Is BTKi or BCL2i preferable as first novel therapy in patients with CLL? The case for BCL2i. Blood Adv. 2022;6(4):1365–70. https://doi.org/10.1182/bloodadvances.2019001205. Provides insights on sequencing of novel therapies in CLL.
•• NCCN: National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: chronic lymphocytic leukemia/small lymphocytic leukemia. 2023 https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Accessed May 8, 2023. Consensus guidelines for the sequencing of therapy in CLL including R/R disease.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10. https://doi.org/10.1056/NEJMoa1313984.
Ahn IE, Farooqui MZH, Tian X, Valdez J, Sun C, Soto S, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood. 2018;131(21):2357–66. https://doi.org/10.1182/blood-2017-12-820910.
Furstenau M, De Silva N, Eichhorst B, Hallek M. Minimal residual disease assessment in CLL: ready for use in clinical routine? Hemasphere. 2019;3(5):e287. https://doi.org/10.1097/HS9.0000000000000287.
• Wierda WG, Rawstron A, Cymbalista F, Badoux X, Rossi D, Brown JR, et al. Measurable residual disease in chronic lymphocytic leukemia: expert review and consensus recommendations. Leukemia. 2021;35(11):3059–72. https://doi.org/10.1038/s41375-021-01241-1. Elucidates the role of MRD in CLL and potential use to determine intensity and duration of therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Oluwatobi Odetola declares that he has no conflict of interest. Shuo Ma has received institutional research funding from AbbVie, Acerta, BeiGene, Gilead, Incyte, Juno, NCCN, Novartis, Pharmacyclics, and TG Therapeutics and has served on advisory boards and delivered lectures for AbbVie, AstraZeneca, Genentech, Gilead, Kite, Janssen, and Pharmacyclics.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Odetola, O., Ma, S. Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL). Curr Hematol Malig Rep 18, 130–143 (2023). https://doi.org/10.1007/s11899-023-00700-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-023-00700-z