Abstract
Purpose of Review
This review describes the recent progress in nuclease-based therapeutic applications for inherited heart diseases in vitro, highlights the development of the most recent genome editing technologies and discusses the associated challenges for clinical translation.
Recent Findings
Inherited cardiovascular disorders are passed from generation to generation. Over the past decade, considerable progress has been made in understanding the genetic basis of inherited heart diseases. The timely emergence of genome editing technologies using engineered programmable nucleases has revolutionized the basic research of inherited cardiovascular diseases and holds great promise for the development of targeted therapies.
Summary
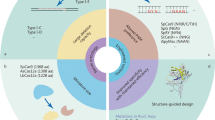
The genome editing toolbox is rapidly expanding, and new tools have been recently added that significantly expand the capabilities of engineered nucleases. Newer classes of versatile engineered nucleases, such as the “base editors,” have been recently developed, offering the potential for efficient and precise therapeutic manipulation of the human genome.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
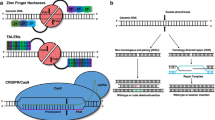
Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A. 1996;93(3):1156–60.
Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326(5959):1509–12.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21.
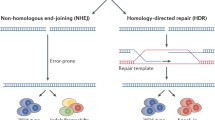
Jasin M, Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harb Perspect Biol. 2013;5(11):a012740.
Chiruvella KK, Liang Z, Wilson TE. Repair of double-strand breaks by end joining. Cold Spring Harb Perspect Biol. 2013;5(5):a012757.
Termglinchan V, Karakikes I, Seeger T et al. Current status of genome editing in cardiovascular medicine. In: Turksen K, editor. Genome editing. Cham: Springer International Publishing; 2016. p. 107–26.
• Karakikes I, et al. Correction of human phospholamban R14del mutation associated with cardiomyopathy using targeted nucleases and combination therapy. Nat Commun. 2015;6:6955. This proof-of-principle study describes how engineered nucleases can be used to correct a pathogenic mutation associated with dilated cardiomyopathy in vitro.
Ang YS, et al. Disease model of GATA4 mutation reveals transcription factor cooperativity in human cardiogenesis. Cell. 2016;167(7):1734–1749 e22.
Liang P, Sallam K, Wu H, Li Y, Itzhaki I, Garg P, et al. Patient-specific and genome-edited induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of Brugada syndrome. J Am Coll Cardiol. 2016;68(19):2086–96.
Karakikes I, Termglinchan V, Cepeda DA, Lee J, Diecke S, Hendel A, et al. A comprehensive TALEN-based knockout library for generating human-induced pluripotent stem cell-based models for cardiovascular diseases. Circ Res. 2017;120(10):1561–71.
Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21(2):121–31.
Pardo B, Gomez-Gonzalez B, Aguilera A. DNA repair in mammalian cells: DNA double-strand break repair: how to fix a broken relationship. Cell Mol Life Sci. 2009;66(6):1039–56.
Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33(5):538–42.
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759–71.
•• Komor AC, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature, 2016;533(7603):420–4. This study demonstrates that genome editing can be used to make precise changes in DNA without causing double-strand breaks.
Yang L, Briggs AW, Chew WL, Mali P, Guell M, Aach J, et al. Engineering and optimising deaminase fusions for genome editing. Nat Commun. 2016;7:13330.
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature. 2017;551(7681):464–71.
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529(7587):490–5.
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351(6268):84–8.
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, et al. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017;550(7676):407–10.
Casini A, Olivieri M, Petris G, Montagna C, Reginato G, Maule G, et al. A highly specific SpCas9 variant is identified by in vivo screening in yeast. Nat Biotechnol. 2018;36(3):265–71.
Hu JH, Miller SM, Geurts MH, Tang W, Chen L, Sun N, et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. In: Nature, vol. 556; 2018. p. 57–63.
Zacchigna S, Zentilin L, Giacca M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ Res. 2014;114(11):1827–46.
Lau CH, Suh Y. In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease. F1000Res. 2017;6:2153.
El Refaey M, et al. In vivo genome editing restores dystrophin expression and cardiac function in dystrophic mice. Circ Res. 2017;121(8):923–9.
Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351(6271):400–3.
• Nelson CE, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351(6271):403–7. In this study, the gene defect responsible for Duchenne muscular dystrophy was corrected in a mouse model by AAV-mediated genome editing in vivo.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
Balpreet Kaur, Isaac Perea Gil, and Ioannis Karakikes declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Regenerative Medicine
Rights and permissions
About this article
Cite this article
Kaur, B., Perea-Gil, I. & Karakikes, I. Recent Progress in Genome Editing Approaches for Inherited Cardiovascular Diseases. Curr Cardiol Rep 20, 58 (2018). https://doi.org/10.1007/s11886-018-0998-3
Published:
DOI: https://doi.org/10.1007/s11886-018-0998-3