Abstract
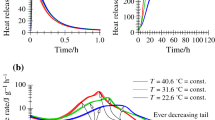
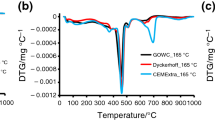
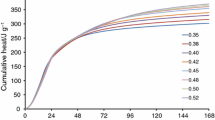
Portland cement have to hydrate in cold climates in some particular conditions. Therefore, a better understanding of cement hydration under low temperatures would benefit the cement-based composites application. In this study, Portland cement was, therefore, kinetically and thermodynamically simulated based on a simple kinetics model and minimization of Gibbs free energy. The results of an evaluation indicate that Portland cement hydration impact factors include the water–cement ratio (w/c), temperature, and specific surface area, with the latter being an especially remarkable factor. Therefore, increasing the specific surface area to an appropriate level may be a solution to speed the delayed hydration due to low temperatures. Meanwhile, the w/c ratio is believed to be controlled under cold climates with consideration of durability. The thermodynamic calculation results suggest that low-temperature influences can be divided into three levels: irrevocable effects (<0 °C), recoverable effects (0–10 °C), and insignificant effects (10–20 °C). Portland cement was additionally measured via X-ray diffraction, thermal gravity analysis, and low-temperature nitrogen adsorption test in a laboratory and comparisons were drawn that validate the simulation result.








Similar content being viewed by others
References
ACI Committee 306 (2010a) ACI 306.1-90, Standard Specification for Cold Weather Concreting, (Reapproved 2002) (ed.) American Concrete Institute, Farmington Hills
ACI Committee 306 (2010b) ACI 306R-10, Guide to Cold Weather Concreting. American Concrete Institute, Farmington Hills
ASTM (2014) C305-14, Standard Practice for Mechanical Mixing of Hydraulic-Cement Pastes and Mortars of Plastic Consistency. ASTM International West Conshohocken, Philadelphia
Azad VJ, Li C, Verba C, Ideker JH, Isgor OB (2016) A COMSOL-GEMS interface for modeling coupled reactive-transport geochemical processes. Comput Geosci. doi:10.1016/j.cageo.2016.04.002
Balonis M, Lothenbach B, Le Saout G, Glasser FP (2010) Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem Concr Res 40:1009–1022. doi:10.1016/j.cemconres.2010.03.002
Beaudoin JJ, Gu P, Marchand J, Tamtsia B, Myers RE, Liu Z (1998) Solvent replacement studies of hydrated Portland cement systems: the role of calcium hydroxide. Adv Cem Based Mater 8:56–65. doi:10.1016/S1065-7355(98)00008-X
Bentz DP (2000) CEMHYD3D: A three-dimensional cement hydration and microstructure development modelling package. Version 2.0. Natl Inst Stand Technol Interag Rep, p 7232
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. doi:10.1021/ja01269a023
Camilleri J (2011) Characterization and hydration kinetics of tricalcium silicate cement for use as a dental biomaterial. Dent Mater 27:836–844. doi:10.1016/j.dental.2011.04.010
Cheung J, Jeknavorian A, Roberts L, Silva D (2011) Impact of admixtures on the hydration kinetics of Portland cement. Cem Concr Res 41:1289–1309. doi:10.1016/j.cemconres.2011.03.005
Demirboğa R, Karagöl F, Polat R, Kaygusuz MA (2014) The effects of urea on strength gaining of fresh concrete under the cold weather conditions. Constr Build Mater 64:114–120. doi:10.1016/j.conbuildmat.2014.04.008
Kozikowski RL, McCall WC, Suprenant BA (2014) Cold weather concreting strategies. William E Rush, Jr, p 45
Kulik DA, Wagner T, Dmytrieva SV, Kosakowski G, Hingerl FF, Chudnenko KV, Berner UR (2013) GEM-Selektor geochemical modeling package: revised algorithm and GEMS3 K numerical kernel for coupled simulation codes. Comput Geosci 17:1–24. doi:10.1007/s10596-012-9310-6
Lee GC, Shih TS, Chang KC (1988) Mechanical properties of concrete at low temperature. J Cold Reg Eng 2:13–24. doi:10.1061/(ASCE)0887-381X(1988)2:1(13)
Lin Feng, Meyer Christian (2009) Hydration kinetics modeling of Portland cement considering the effects of curing temperature and applied pressure. Cem Concr Res 39:255–265. doi:10.1016/j.cemconres.2009.01.014
Liu J, Li Y, Ouyang P, Yang Y (2015) Hydration of the silica fume-Portland cement binary system at lower temperature. Constr Build Mater 93:919–925. doi:10.1016/j.conbuildmat.2015.05.069
Lothenbach B, Winnefeld F (2006) Thermodynamic modelling of the hydration of Portland cement. Cem Concr Res 36:209–226. doi:10.1016/j.cemconres.2005.03.001
Lothenbach B, Matschei T, Möschner G, Glasser FP (2008) Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem Concr Res 38:1–18. doi:10.1016/j.cemconres.2007.08.017
Maslehuddin M, Page CL, Rasheeduzzafar (1997) Temperature effect on the pore solution chemistry in contaminated cements. Mag Concr Res 49:5–14. doi:10.1680/macr.1997.49.178.5
Nurse RW (1949) Steam curing of concrete. Mag Concr Res 1:79–88. doi:10.1680/macr.1949.1.2.79
Oey T, Stoian J, Li J, Vong C, Balonis M, Kumar A, Franke W, Sant G (2014) Comparison of Ca(NO3)2 and CaCl2 admixtures on reaction, setting, and strength evolutions in plain and blended cementing formulations. J Mater Civ Eng. doi:10.1061/(ASCE)MT.1943-5533.0001240
Pade C, Guimaraes M (2007) The CO2 uptake of concrete in a 100 year perspective. Cem Concr Res 37:1348–1356. doi:10.1016/j.cemconres.2007.06.009
Parrot LJ, Killoh DC (1984) Prediction of cement hydration. In: Proceedings of the British Ceramics Society, p 41
Polat R (2016) The effect of antifreeze additives on fresh concrete subjected to freezing and thawing cycles. Cold Reg Sci Technol 127:10–17. doi:10.1016/j.coldregions.2016.04.008
Powers TC, Brownyard TL (1946) Studies of the physical properties of hardened Portland cement paste. In: ACI Journal Proceedings. ACI
Qiao Y, Wang H, Cai L, Zhang W, Yang B (2016) Influence of low temperature on dynamic behavior of concrete. Constr Build Mater 115:214–220. doi:10.1016/j.conbuildmat.2016.04.046
Saul AGA (1951) Principles underlying the steam curing of concrete at atmospheric pressure. Mag Concr Res 2:127–140. doi:10.1680/macr.1951.2.6.127
Thomas JJ, Biernacki JJ, Bullard JW, Bishnoi S, Dolado JS, Scherer GW, Luttge A (2011) Modeling and simulation of cement hydration kinetics and microstructure development. Cem Concr Res 41:1257–1278. doi:10.1016/j.cemconres.2010.10.004
Tomosawa F (1997) Development of a kinetic model for hydration of cement. In: Proceedings of the Tenth International Congress Chemistry of Cement, Gothenburg, p 5158
Van Breugel K (1995) Numerical simulation of hydration and microstructural development in hardening cement-based materials:(II) applications. Cem Concr Res 25:522–530. doi:10.1016/0008-8846(95)00041-A
Xu L, Wang P, Zhang G (2012) Formation of ettringite in Portland cement/calcium aluminate cement/calcium sulfate ternary system hydrates at lower temperatures. Constr Build Mater 31:347–352. doi:10.1016/j.conbuildmat.2011.12.078
Young JF, Mindess S, Darwin D (2002) Concrete. Prentice Hall
Acknowledgements
This study was financially supported by the National Key Technology R&D Program, China (2014BAG05B04), the Doctoral Postgraduate Technical Project of Chang’an University (2014G5210006). The main experiments were conducted in the Key Laboratory for Special Area Highway Engineering, Ministry of Education of China, and the Materials Analysis Center, School of Materials Science and Engineering, Chang’an University, as well as the Xi’an Mineral Resources Surveillance and Test Center, Ministry of Land and Resource of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Z., Sha, A., Hu, L. et al. Kinetic and thermodynamic modeling of Portland cement hydration at low temperatures. Chem. Pap. 71, 741–751 (2017). https://doi.org/10.1007/s11696-016-0007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0007-5