Abstract
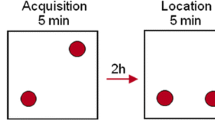
Adjuvant chemotherapy has been used for decades to treat cancer, and it is well known that disruptions in cognitive function and memory are common chemotherapeutic adverse effects. However, studies using neuropsychological metrics have also reported group differences in cognitive function and memory before or without chemotherapy, suggesting that complex factors obscure the true etiology of chemotherapy-induced cognitive dysfunction (CICD) in humans. Therefore, to better understand possible mechanisms of CICD, we explored the effects of CICD in rats through cognition testing using novel object recognition (NOR) and contextual fear conditioning (CFC), and through metabolic neuroimaging via [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET). Cancer-naïve, female Sprague-Dawley rats were administered either saline (1 mL/kg) or doxorubicin (DOX) (1 mg/kg in a volume of 1 mL/kg) weekly for five weeks (total dose = 5 mg/kg), and underwent cognition testing and PET imaging immediately following the treatment regime and 30 days post treatment. We did not observe significant differences with CFC testing post-treatment for either group. However, the chemotherapy group exhibited significantly decreased performance in the NOR test and decreased 18F-FDG uptake only in the prefrontal cortex 30 days post-treatment. These results suggest that long-term impairment within the prefrontal cortex is a plausible mechanism of CICD in this study, suggesting DOX-induced toxicity in the prefrontal cortex at the dose used.



Similar content being viewed by others
References
Abraham, J., Haut, M. W., Moran, M. T., Filburn, S., Lemiuex, S., & Kuwabara, H. (2008). Adjuvant chemotherapy for breast cancer: effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer, 8(1), 88–91.
Ahles, T. A., & Saykin, A. J. (2007). Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer, 7(3), 192–201.
Ahles, T. A., Saykin, A. J., Furstenberg, C. T., Cole, B., Mott, L. A., Skalla, K., et al. (2002). Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol, 20(2), 485–493.
Ahles, T. A., Saykin, A. J., McDonald, B. C., Furstenberg, C. T., Cole, B. F., Hanscom, B. S., et al. (2008). Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat, 110(1), 143–152.
Anagnostaras, S. G., Maren, S., & Fanselow, M. S. (1995). Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem, 64(3), 191–194.
Anagnostaras, S. G., Maren, S., & Fanselow, M. S. (1999). Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci, 19(3), 1106–1114.
Anderson-Hanley, C., Sherman, M. L., Riggs, R., Agocha, V. B., & Compas, B. E. (2003). Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc, 9(7), 967–982.
Bender, C. M., Sereika, S. M., Berga, S. L., Vogel, V. G., Brufsky, A. M., Paraska, K. K., & Ryan, C. M. (2006). Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology, 15(5), 422–430.
Bevins, R. A., & Besheer, J. (2006). Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc, 1(3), 1306–1311.
Bloom, A. S., LaViolette, P. S., Chitambar, C. R., Collier, W., Durgerian, S. J., Kalyanaraman, B., et al. (2010). Effects of doxorubicin on brain activity and functional connectivity in rats. Proc Int Soc Magn Reson Med, 18, 1229.
Boles Ponto, L. L., Menda, Y., Magnotta, V. A., Yamada, T. H., Denburg, N. L., & Schultz, S. K. (2015). Frontal hypometabolism in elderly breast cancer survivors determined by [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET): a pilot study. Int J Geriatr Psychiatry, 30(6), 587–594.
Brezden, C. B., Phillips, K.-A., Abdolell, M., Bunston, T., & Tannock, I. F. (2000). Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol, 18(14), 2695–2701.
Byun, N. E., Grannan, M., Bubser, M., Barry, R. L., Thompson, A., Rosanelli, J., et al. (2014). Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology, 39(7), 1578–1593.
Christie, L.-A., Acharya, M. M., Parihar, V. K., Nguyen, A., Martirosian, V., & Limoli, C. L. (2012). Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res, 18(7), 1954–1965.
de Ruiter, M. B., Reneman, L., Boogerd, W., Veltman, D. J., van Dam, F. S. A. M., Nederveen, A. J., et al. (2011). Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp, 32(8), 1206–1219.
Deprez, S., Amant, F., Smeets, A., Peeters, R., Leemans, A., Van Hecke, W., et al. (2012). Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol, 30(3), 274–281.
Ennaceur, A., & Delacour, J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res, 31(1), 47–59.
Ennaceur, A., & Meliani, K. (1992). A new one-trial test for neurobiological studies of memory in rats. III. Spatial vs. non-spatial working memory. Behavioural Brain Research, 51(1), 83–92.
Freeman, J. R., & Broshek, D. K. (2002). Assessing cognitive dysfunction in breast cancer: what are the tools? Clin Breast Cancer, 3(Suppl 3), S91–S99.
Fremouw, T., Fessler, C. L., Ferguson, R. J., & Burguete, Y. (2012). Preserved learning and memory in mice following chemotherapy: 5-Fluorouracil and doxorubicin single agent treatment, doxorubicin-cyclophosphamide combination treatment. Behav Brain Res, 226(1), 154–162.
Grayson, B., Idris, N. F., & Neill, J. C. (2007). Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res, 184(1), 31–38.
Hede, K. (2008). Chemobrain is real but may need new name. J Natl Cancer Inst, 100(3), 162–3,169.
Hess, L. M., & Insel, K. C. (2007). Chemotherapy-related change in cognitive function: a conceptual model. Oncol Nurs Forum, 34(5), 981–994.
Hurria, A., Somlo, G., & Ahles, T. (2007). Renaming “chemobrain.”. Cancer Invest, 25(6), 373–377.
Jansen, C. E., Cooper, B. A., Dodd, M. J., & Miaskowski, C. A. (2011). A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer, 19(10), 1647–1656.
Jarrett, B. L. (2013). Chemotherapy induced deficits in cognition and affective behavior. Ph.D. Thesis, Ohio State University, Neuroscience Graduate Studies Program.
Jim, H. S. L., Phillips, K. M., Chait, S., Faul, L. A., Popa, M. A., Lee, Y.-H., et al. (2012). Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol, 30(29), 3578–3587.
Kaiser, J., Bledowski, C., & Dietrich, J. (2014). Neural correlates of chemotherapy-related cognitive impairment. Cortex, 54, 33–50.
Kesler, S. R. (2014). Default mode network as a potential biomarker of chemotherapy-related brain injury. Neurobiol Aging, 35(Suppl 2), S11–S19.
Kesler, S. R., Kent, J. S., & O'Hara, R. (2011). Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol, 68(11), 1447–1453.
Liedke, P. E. R., Reolon, G. K., Kilpp, B., Brunetto, A. L., Roesler, R., & Schwartsmann, G. (2009). Systemic administration of doxorubicin impairs aversively motivated memory in rats. Pharmacol Biochem Behav, 94(2), 239–243.
Loening, A. M., & Gambhir, S. S. (2003). AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging, 2(3), 131–137.
Maren, S., & Fanselow, M. S. (1997). Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem, 67(2), 142–149.
Maren, S., Aharonov, G., & Fanselow, M. S. (1997). Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res, 88(2), 261–274.
McAllister, T. W., Ahles, T. A., Saykin, A. J., Ferguson, R. J., McDonald, B. C., Lewis, L. D., et al. (2004). Cognitive effects of cytotoxic cancer chemotherapy: predisposing risk factors and potential treatments. Curr Psychiatry Rep, 6(5), 364–371.
McDonald, B. C., Conroy, S. K., Ahles, T. A., West, J. D., & Saykin, A. J. (2010). Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat, 123(3), 819–828.
McDonald, B. C., Conroy, S. K., Ahles, T. A., West, J. D., & Saykin, A. J. (2012). Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol, 30(20), 2500–2508.
Meyers, C. A., & Scheibel, R. S. (1990). Early detection and diagnosis of neurobehavioral disorders associated with cancer and its treatment. Oncology (Williston Park), 4(7), 115–122 126–127,130.
Meyers, C. A., Byrne, K. S., & Komaki, R. (1995). Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer, 12(3), 231–235.
Minisini, A., Atalay, G., Bottomley, A., Puglisi, F., Piccart, M., & Biganzoli, L. (2004). What is the effect of systemic anticancer treatment on cognitive function? Lancet Oncol, 5(5), 273–282.
Momparler, R. L., Karon, M., Siegel, S. E., & Avila, F. (1976). Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res, 36(8), 2891–2895.
Newhouse, P., & Dumas, J. (2015). Estrogen-cholinergic interactions: implications for cognitive aging. Horm Behav, 74, 173–185.
Nudelman, K. N. H., Wang, Y., McDonald, B. C., Conroy, S. K., Smith, D. J., West, J. D., et al. (2014). Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: a prospective study using pulsed arterial spin labeling MRI perfusion. PLoS One, 9(5), e96713.
O’Shaughnessy, J. A. (2002). Effects of epoetin alfa on cognitive function, mood, asthenia, and quality of life in women with breast cancer undergoing adjuvant chemotherapy. Clin Breast Cancer, 3(Suppl 3), S116–S120.
Rubins, D. J., Melega, W. P., Lacan, G., Way, B., Plenevaux, A., Luxen, A., & Cherry, S. R. (2003). Development and evaluation of an automated atlas-based image analysis method for microPET studies of the rat brain. Neuroimage, 20(4), 2100–2118.
Saykin, A. J., Ahles, T. A., Schoenfeld, J. D., Wishart, H. A., Pietras, C. G., Furstenberg, C. T., et al. (2003). Gray matter reduction on voxel-based morphometry in chemotherapy-treated cancer survivors. J Int Neuropsychol Soc, 9(2), 246.
Saykin, A. J., de Ruiter, M. B., McDonald, B. C., Deprez, S., & Silverman, D. H. S. (2013). Neuroimaging biomarkers and cognitive function in non-CNS cancer and its treatment: current status and recommendations for future research. Brain Imaging Behav, 7(4), 363–373.
Schagen, S. B., van Dam, F. S. A. M., Muller, M. J., Boogerd, W., Lindeboom, J., & Bruning, P. F. (1999). Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer, 85(3), 640–650.
Schagen, S. B., Das, E., & van Dam, F. S. A. M. (2009). The influence of priming and pre-existing knowledge of chemotherapy-associated cognitive complaints on the reporting of such complaints in breast cancer patients. Psychooncology, 18(6), 674–678.
Schilder, C. M., Seynaeve, C., Beex, L. V., Boogerd, W., Linn, S. C., Gundy, C. M., et al. (2010a). Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol, 28(8), 1294–1300.
Schilder, C. M. T., Seynaeve, C., Linn, S. C., Boogerd, W., Beex, L. V. A. M., Gundy, C. M., et al. (2010b). Cognitive functioning of postmenopausal breast cancer patients before adjuvant systemic therapy, and its association with medical and psychological factors. Crit Rev Oncol Hematol, 76(2), 133–141.
Schilder, C. M., Seynaeve, C., Linn, S. C., Boogerd, W., Gundy, C. M., Beex, L. V., et al. (2010c). The impact of different definitions and reference groups on the prevalence of cognitive impairment: a study in postmenopausal breast cancer patients before the start of adjuvant systemic therapy. Psychooncology, 19(4), 415–422.
Seigers, R., & Fardell, J. E. (2011). Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neuroscience and Biobehavioral Reviews, 35(3), 729–741.
Seigers, R., Schagen, S. B., Van Tellingen, O., & Dietrich, J. (2013). Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging and Behavior, 7(4), 453–459.
Seigers, R., Loos, M., Van Tellingen, O., Boogerd, W., Smit, A. B., & Schagen, S. B. (2015). Cognitive impact of cytotoxic agents in mice. Psychopharmacology, 232(1), 17–37.
Selamat, M. H., Loh, S. Y., Mackenzie, L., & Vardy, J. (2014). Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PloS One, 9(9), e108002.
Silberfarb, P. M., Philibert, D., & Levine, P. M. (1980). Psychosocial aspects of neoplastic disease: II. Affective and cognitive effects of chemotherapy in cancer patients. The American Journal of Psychiatry, 137(5), 597–601.
Silverman, D. H. S., Dy, C. J., Castellon, S. A., Lai, J., Pio, B. S., Abraham, L., et al. (2007). Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Research and Treatment, 103(3), 303–311.
Stemmer, S. M., Stears, J. C., Burton, B. S., Jones, R. B., & Simon, J. H. (1994). White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. AJNR. American Journal of Neuroradiology, 15(7), 1267–1273.
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. (2005). Guidance for industry: Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. MD: Rockville http://www.fda.gov/downloads/Guidances/UCM078932.pdf.
van Dam, F. S. A. M., Schagen, S. B., Muller, M. J., Boogerd, W., van der Wall, E., Droogleever Fortuyn, M. E., & Rodenhuis, S. (1998). Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. Journal of the National Cancer Institute, 90(3), 210–218.
Vardy, J. (2009). Cognitive function in breast cancer survivors. Cancer Treatment and Research, 151, 387–419.
Vardy, J., Rourke, S., & Tannock, I. F. (2007). Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. Journal of Clinical Oncology, 25(17), 2455–2463.
Von Ah, D., Storey, S., Jansen, C. E., & Allen, D. H. (2013). Coping strategies and interventions for cognitive changes in patients with cancer. Seminars in Oncology Nursing, 29(4), 288–299.
Wallenstein, G. V., & Vago, D. R. (2001). Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiology of Learning and Memory, 75(3), 245–252.
Watson, D. J. G., Loiseau, F., Ingallinesi, M., Millan, M. J., Marsden, C. A., & Fone, K. C. F. (2012). Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology, 37(3), 770–786.
Wefel, J. S., Kayl, A. E., & Meyers, C. A. (2004). Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. British Journal of Cancer, 90(9), 1691–1696.
Weiss, B. (2008). Chemobrain: a translational challenge for neurotoxicology. Neurotoxicology, 29(5), 891–898.
Wieneke, M. H., & Dienst, E. R. (1995). Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology, 4(1), 61–66.
Acknowledgments
This research was supported by the American Cancer Society (IRG-58-009-51 to C.C.Q.). The authors thank Dr. M. Scott Thompson at the Vanderbilt University Medical Center Oncology Pharmacy; Dr. Randy L. Smith Barrett at the Vanderbilt University School of Medicine Rat Neurobehavioral Core; and Fuxue Xin and Dr. Zou Yue at the Vanderbilt University Institute of Imaging Science Center for Small Animal Imaging. We also acknowledge Mackenzie Whitehurst for her assistance in analyzing the novel object recognition data and Tanner Liddy for his assistance in analyzing the contextual fear conditioning data. Finally, we thank Mrs. Joan M. Gee for asking the question “I wonder what chemotherapy will do to my brain?”; her personal experiences with breast cancer and chemotherapy in 2010 was the motivation for this study.
Author contributions
Concept and study design: Barry, Byun, Gee, Quarles
Development of methodology: Barry, Byun, Tantawy, Wilson
Acquisition of data: Barry, Byun, Tantawy, Wilson, Gee
Analysis and interpretation of data: Barry, Byun, Tantawy, Mackey, Flom, Stark
Writing, reviewing, and revising manuscript: Barry, Byun, Tantawy, Quarles
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing financial interests
The authors declare no competing financial interests.
Additional information
Robert L. Barry and Nellie E. Byun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Barry, R.L., Byun, N.E., Tantawy, M.N. et al. In vivo neuroimaging and behavioral correlates in a rat model of chemotherapy-induced cognitive dysfunction. Brain Imaging and Behavior 12, 87–95 (2018). https://doi.org/10.1007/s11682-017-9674-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9674-2