Abstract
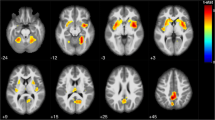
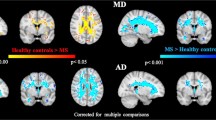
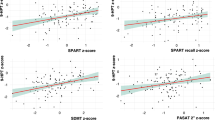
Cognitive impairment (CI), mainly involving attention and processing speed (A-PS), is a common and disabling symptom in multiple sclerosis (MS). Symbol Digit Modalities Test (SDMT) is one of the more sensitive and reliable tests to assess A-PS deficits in MS. Structural MRI correlates of A-PS in MS still need to be clarified. This study aimed to investigate, in a large group of MS patients, the relationship between regional gray matter (GM) atrophy and SDMT performance. 125 relapsing remitting MS patients and 52 healthy controls (HC) underwent a 3 T–MRI protocol including high-resolution 3D–T1 imaging. All subjects underwent a neurological evaluation and SDMT. A Voxel Based Morphometry analysis was performed to assess: 1) correlations between regional GM volume and SDMT performance in MS patients; 2) regional differences in GM volume between MS patients and HC. Thalamic, putamen and cerebellar volumes were also calculated using FIRST tool from the FMRIB Software Library. A linear regression analysis was performed to assess the contribution of each one of these structures to A-PS performance. A significant negative correlation was found between regional GM volume and SDMT score at the level of the thalamus, cerebellum, putamen, and occipital cortex in MS patients. Thalamus, cerebellum and putamen also showed significant GM atrophy in MS patients compared to HC. Thalamic atrophy is also an independent and additional contributor to A-PS deficits in MS patients. These findings support the role of thalamus as the most relevant GM structure subtending A-PS performance in MS, as measured by SDMT.


Similar content being viewed by others
References
Amato, M. P., Portaccio, E., Goretti, B., Zipoli, V., Ricchiuti, L., De Caro, M. F., et al. (2006). The Rao's brief repeatable battery and Stroop test: normative values with age, education and gender corrections in an Italian population. Multiple Sclerosis, 12(6), 787–793.
Amato, M. P., Portaccio, E., Goretti, B., Zipoli, V., Iudice, A., Della Pina, D., et al. (2010). Relevance of cognitive deterioration in early relapsing-remitting MS: a 3-year follow-up study. Multiple Sclerosis, 16(12), 1474–1482. doi:10.1177/1352458510380089.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. doi:10.1016/j.neuroimage.2007.07.007.
Batista, S., Zivadinov, R., Hoogs, M., Bergsland, N., Heininen-Brown, M., Dwyer, M. G., et al. (2012). Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. Journal of Neurology, 259(1), 139–146. doi:10.1007/s00415-011-6147-1.
Battaglini, M., Jenkinson, M., & De Stefano, N. (2012). Evaluating and reducing the impact of white matter lesions on brain volume measurements. Human Brain Mapping, 33(9), 2062–2071. doi:10.1002/hbm.21344.
Benedict, R. H., & Zivadinov, R. (2011). Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nature Reviews. Neurology, 7(6), 332–342. doi:10.1038/nrneurol.2011.61.
Benedict, R. H., Duquin, J. A., Jurgensen, S., Rudick, R. A., Feitcher, J., Munschauer, F. E., et al. (2008). Repeated assessment of neuropsychological deficits in multiple sclerosis using the symbol digit modalities test and the MS neuropsychological screening questionnaire. Multiple Sclerosis, 14(7), 940–946. doi:10.1177/1352458508090923.
Benedict, R. H., Hulst, H. E., Bergsland, N., Schoonheim, M. M., Dwyer, M. G., Weinstock-Guttman, B., et al. (2013). Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Multiple Sclerosis, 19(11), 1478–1484. doi:10.1177/1352458513478675.
Bergsland, N., Zivadinov, R., Dwyer, M. G., Weinstock-Guttman, B., & Benedict, R. H. (2016). Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Multiple Sclerosis, 22(10), 1327–1336. doi:10.1177/1352458515616204.
Bisecco, A., Rocca, M. A., Pagani, E., Mancini, L., Enzinger, C., Gallo, A., et al. (2015). Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Human Brain Mapping. doi:10.1002/hbm.22809.
Bonnet, M. C., Allard, M., Dilharreguy, B., Deloire, M., Petry, K. G., & Brochet, B. (2010). Cognitive compensation failure in multiple sclerosis. Neurology, 75(14), 1241–1248. doi:10.1212/WNL.0b013e3181f612e3.
Ceccarelli, A., Jackson, J. S., Tauhid, S., Arora, A., Gorky, J., Dell'Oglio, E., et al. (2012). The impact of lesion in-painting and registration methods on voxel-based morphometry in detecting regional cerebral gray matter atrophy in multiple sclerosis. AJNR. American Journal of Neuroradiology, 33(8), 1579–1585. doi:10.3174/ajnr.A3083.
Cerasa, A., Valentino, P., Chiriaco, C., Pirritano, D., Nistico, R., Gioia, C. M., et al. (2013). MR imaging and cognitive correlates of relapsing-remitting multiple sclerosis patients with cerebellar symptoms. Journal of Neurology, 260(5), 1358–1366. doi:10.1007/s00415-012-6805-y.
Chiaravalloti, N. D., & DeLuca, J. (2008). Cognitive impairment in multiple sclerosis. Lancet Neurology, 7(12), 1139–1151. doi:10.1016/S1474-4422(08)70259-X.
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. doi:10.1006/nimg.1998.0395.
Damasceno, A., Damasceno, B. P., & Cendes, F. (2014). The clinical impact of cerebellar grey matter pathology in multiple sclerosis. PloS One, 9(5), e96193. doi:10.1371/journal.pone.0096193.
DeLuca, G. C., Yates, R. L., Beale, H., & Morrow, S. A. (2015). Cognitive impairment in multiple sclerosis: clinical, radiologic and pathologic insights. Brain Pathology, 25(1), 79–98. doi:10.1111/bpa.12220.
Drake, A. S., Weinstock-Guttman, B., Morrow, S. A., Hojnacki, D., Munschauer, F. E., & Benedict, R. H. (2010). Psychometrics and normative data for the multiple sclerosis functional composite: replacing the PASAT with the symbol digit modalities test. Multiple Sclerosis, 16(2), 228–237. doi:10.1177/1352458509354552.
Genova, H. M., Hillary, F. G., Wylie, G., Rypma, B., & Deluca, J. (2009). Examination of processing speed deficits in multiple sclerosis using functional magnetic resonance imaging. Journal of the International Neuropsychological Society, 15(3), 383–393. doi:10.1017/S1355617709090535.
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J., & Frackowiak, R. S. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14(1 Pt 1), 21–36. doi:10.1006/nimg.2001.0786.
Houtchens, M. K., Benedict, R. H., Killiany, R., Sharma, J., Jaisani, Z., Singh, B., et al. (2007). Thalamic atrophy and cognition in multiple sclerosis. Neurology, 69(12), 1213–1223. doi:10.1212/01.wnl.0000276992.17011.b5.
Julian, L. J. (2011). Cognitive functioning in multiple sclerosis. Neurologic Clinics, 29(2), 507–525. doi:10.1016/j.ncl.2010.12.003.
Kurtzke, J. F. (1983). Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology, 33(11), 1444–1452.
Lansley, J., Mataix-Cols, D., Grau, M., Radua, J., & Sastre-Garriga, J. (2013). Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neuroscience and Biobehavioral Reviews, 37(5), 819–830. doi:10.1016/j.neubiorev.2013.03.006.
Lopez-Gongora, M., Querol, L., & Escartin, A. (2015). A one-year follow-up study of the symbol digit modalities test (SDMT) and the paced auditory serial addition test (PASAT) in relapsing-remitting multiple sclerosis: an appraisal of comparative longitudinal sensitivity. BMC Neurology, 15, 40. doi:10.1186/s12883-015-0296-2.
Middleton, F. A., & Strick, P. L. (2000). Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research. Brain Research Reviews, 31(2–3), 236–250.
Minagar, A., Barnett, M. H., Benedict, R. H., Pelletier, D., Pirko, I., Sahraian, M. A., et al. (2013). The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology, 80(2), 210–219. doi:10.1212/WNL.0b013e31827b910b.
Morrow, S. A., Drake, A., Zivadinov, R., Munschauer, F., Weinstock-Guttman, B., & Benedict, R. H. (2010). Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. The Clinical Neuropsychologist, 24(7), 1131–1145. doi:10.1080/13854046.2010.511272.
Nocentini, U., Bozzali, M., Spano, B., Cercignani, M., Serra, L., Basile, B., et al. (2014). Exploration of the relationships between regional grey matter atrophy and cognition in multiple sclerosis. Brain Imaging and Behavior, 8(3), 378–386. doi:10.1007/s11682-012-9170-7.
Parmenter, B. A., Weinstock-Guttman, B., Garg, N., Munschauer, F., & Benedict, R. H. (2007). Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Multiple Sclerosis, 13(1), 52–57.
Patenaude, B., Smith, S. M., Kennedy, D. N., & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. doi:10.1016/j.neuroimage.2011.02.046.
Polman, C. H., Reingold, S. C., Banwell, B., Clanet, M., Cohen, J. A., Filippi, M., et al. (2011). Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology, 69(2), 292–302. doi:10.1002/ana.22366.
Prinster, A., Quarantelli, M., Orefice, G., Lanzillo, R., Brunetti, A., Mollica, C., et al. (2006). Grey matter loss in relapsing-remitting multiple sclerosis: a voxel-based morphometry study. NeuroImage, 29(3), 859–867. doi:10.1016/j.neuroimage.2005.08.034.
Rao, S. M., Leo, G. J., Bernardin, L., & Unverzagt, F. (1991a). Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology, 41(5), 685–691.
Rao, S. M., Leo, G. J., Ellington, L., Nauertz, T., Bernardin, L., & Unverzagt, F. (1991b). Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology, 41(5), 692–696.
Rao, S. M., Martin, A. L., Huelin, R., Wissinger, E., Khankhel, Z., Kim, E., et al. (2014). Correlations between MRI and information processing speed in MS: a meta-analysis. Multiple Sclerosis International, 2014, 975803. doi:10.1155/2014/975803.
Rocca, M. A., Valsasina, P., Meani, A., Falini, A., Comi, G., & Filippi, M. (2016). Impaired functional integration in multiple sclerosis: a graph theory study. Brain Structure & Function, 221(1), 115–131. doi:10.1007/s00429-014-0896-4.
Sarica, A., Cerasa, A., & Quattrone, A. (2015). The neurocognitive profile of the cerebellum in multiple sclerosis. International Journal of Molecular Sciences, 16(6), 12185–12198. doi:10.3390/ijms160612185.
Sastre-Garriga, J., Arevalo, M. J., Renom, M., Alonso, J., Gonzalez, I., Galan, I., et al. (2009). Brain volumetry counterparts of cognitive impairment in patients with multiple sclerosis. Journal of the Neurological Sciences, 282(1–2), 120–124. doi:10.1016/j.jns.2008.12.019.
Sastre-Garriga, J., Alonso, J., Renom, M., Arevalo, M. J., Gonzalez, I., Galan, I., et al. (2011). A functional magnetic resonance proof of concept pilot trial of cognitive rehabilitation in multiple sclerosis. Multiple Sclerosis, 17(4), 457–467. doi:10.1177/1352458510389219.
Schoonheim, M. M., Popescu, V., Rueda Lopes, F. C., Wiebenga, O. T., Vrenken, H., Douw, L., et al. (2012). Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology, 79(17), 1754–1761. doi:10.1212/WNL.0b013e3182703f46.
Smith, A. (1982). Symbol digits modalities test: Manual. Los Angeles: Western Psychological Services.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. doi:10.1002/hbm.10062.
Smith, S. M., Zhang, Y., Jenkinson, M., Chen, J., Matthews, P. M., Federico, A., et al. (2002). Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage, 17(1), 479–489.
Strober, L. B., Christodoulou, C., Benedict, R. H., Westervelt, H. J., Melville, P., Scherl, W. F., et al. (2012). Unemployment in multiple sclerosis: the contribution of personality and disease. Multiple Sclerosis, 18(5), 647–653. doi:10.1177/1352458511426735.
Tedesco, A. M., Chiricozzi, F. R., Clausi, S., Lupo, M., Molinari, M., & Leggio, M. G. (2011). The cerebellar cognitive profile. Brain, 134(Pt 12), 3672–3686. doi:10.1093/brain/awr266.
Weier, K., Penner, I. K., Magon, S., Amann, M., Naegelin, Y., Andelova, M., et al. (2014). Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PloS One, 9(1), e86916. doi:10.1371/journal.pone.0086916.
Zipoli, V., Goretti, B., Hakiki, B., Siracusa, G., Sorbi, S., Portaccio, E., et al. (2010). Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Multiple Sclerosis, 16(1), 62–67. doi:10.1177/1352458509350311.
Acknowledgements
The authors thank all subjects, especially the patients with MS for the time and effort devoted to this study; Antonella Paccone, MRI Center “SUN-FISM,” University of Campania “Luigi Vanvitelli” and Institute of Diagnosis and Care “Hermitage-Capodimonte,” Naples, Italy for technical support; Mattia Siciliano, Department of Medical, Surgical, Neurological, Metabolic and Aging Sciences, University of Campania “Luigi Vanvitelli”, Italy for contributing to statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
Alvino Bisecco, Svetlana Stamenova, Giuseppina Caiazzo, Alessandro d’Ambrosio, Rosaria Sacco, Renato Docimo, Sabrina Esposito, Mario Cirillo and Fabrizio Esposito have no disclosures. Simona Bonavita received speakers’ honoraria from Biogen Idec, Novartis, and Merck-Serono. Gioacchino Tedeschi has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck Serono, and Fondazione Italiana Sclerosi Multipla. Antonio Gallo received honoraria for speaking and travel grants from Biogen, Sanofi-Aventis, Merck Serono, Genzyme, Teva, Bayer-Schering and Novartis.
Ethical approval
This study was approved by the by the local Ethic Committee. All procedures performed in this study involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Alvino Bisecco and Svetlana Stamenova contributed equally to the development of the study.
Rights and permissions
About this article
Cite this article
Bisecco, A., Stamenova, S., Caiazzo, G. et al. Attention and processing speed performance in multiple sclerosis is mostly related to thalamic volume. Brain Imaging and Behavior 12, 20–28 (2018). https://doi.org/10.1007/s11682-016-9667-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9667-6