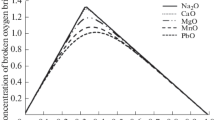
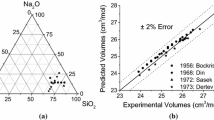
Abstract
Structures of molten oxides (including silicates) strongly affect their physical properties. Although structural analysis is typically performed experimentally by nuclear magnetic resonance and Raman spectroscopy or theoretically by employing thermodynamic quasichemical and cell models, these techniques are time-consuming, and their results are not sufficiently accurate. In this study, high-precision structural evaluations were performed within a relatively short timeframe by combining impedance measurements with the quasichemical model. For this purpose, correlation equations, including the thermodynamic parameters of the quasichemical model and equivalent circuit components obtained by impedance measurements, were derived. Using the proposed method, the evaluation accuracy was improved by approximately 3.6 times as compared with the data generated by FactSage software.







Similar content being viewed by others
References
K.C. Mills, A.B. Fox, ISIJ Int. 43, 1479–1486 (2003)
K. Raj, K.K. Prasad, N.K. Bansal, Nucl. Eng. Des. 236, 914–930 (2006)
K.C. Mills, ISIJ Int. 33, 148–155 (1993)
T. Iida, H. Sakai, Y. Kita, K. Shigeno, ISIJ Int. 40, S110–S114 (2000)
M. Chen, D. Zhang, M. Kou, B. Zhao, ISIJ Int. 54, 2025–2030 (2014)
L. Shartsis, S. Spinner, W. Capps, J. Am. Ceram. Soc. 35, 155–160 (1952)
E.F. Riebling, J. Chem. Phys. 39, 3022–3030 (1963)
Y.E. Lee, D.R. Gaskell, Metall. Trans. 5, 853–860 (1974)
W.D. Kingery, J. Am. Ceram. Soc. 42, 6–10 (1959)
M. Nakamoto, A. Kiyose, T. Tanaka, L. Holappa, M. Hamalainen, ISIJ Int. 47, 38–43 (2007)
H. Hasegawa, T. Kowatari, Y. Shiroki, H. Shibata, H. Ohta, Y. Waseda, Metall. Mater. Trans. B 43B, 1413–1419 (2012)
Y. Kim, K. Morita, ISIJ Int. 54, 2077–2083 (2014)
H. Maekawa, T. Maekawa, K. Kawamura, T. Yokokawa, J. Phys. Chem. 95, 6822–6827 (1991)
S. Sukenaga, K. Kanehashi, H. Shibata, N. Saito, K. Nakashima, Metall. Mater. Trans. B 47B, 2177–2181 (2016)
D.R. Neuville, Chem. Geol. 229, 28–41 (2006)
B. Hehlen, D.R. Neuville, J. Phys. Chem. B 119, 4093–4098 (2015)
A.D. Pelton, M. Blander, Metall. Trans. B 17, 805–815 (1986)
Y. Sasaki, K. Ishii, Tetsu-to-Hagane 88, 419–429 (2002)
S. Sukenaga, T. Nagahisa, K. Kanehashi, N. Saito, K. Nakashima, ISIJ Int. 51, 333–335 (2011). https://doi.org/10.2355/isijinternational.51.333
A.A. Ariskin, V.B. Polyakov, Geochem. Int. 46, 467–486 (2008)
N. Saito, K. Kusada, S. Sukenaga, Y. Ohta, K. Nakashima, ISIJ Int. 52, 2123–2129 (2012)
Y. Harada, K. Kusada, S. Sukenaga, H. Yamamura, Y. Ueshima, T. Mizoguchi, N. Saito, K. Nakashima, ISIJ Int. 54, 2071–2076 (2014)
Y. Harada, S. Sakaguchi, T. Mizoguchi, N. Saito, K. Nakashima, ISIJ Int. 57, 1313–1318 (2017)
Y. Harada, N. Saito, K. Nakashima, ISIJ Int. 57, 23–30 (2017)
Y. Harada, N. Saito, K. Nakashima, ISIJ Int. 59, 421–426 (2019)
A. Romero-Serrano, A.D. Pelton, Metall. Mater. Trans. B 26B, 305–315 (1995)
A.D. Pelton, S.A. Degterov, G. Eriksson, C. Robelin, Y. Dessureault, Metall. Mater. Trans. B 31B, 651–659 (2000)
A.D. Pelton, P. Chartrand, Metall. Mater. Trans. A 32A, 1355–1360 (2001)
C.W. Bale, E. Belisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.H. Jung, Y.B. Kang, J. Melancon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, M.A. Van Ende, CALPHAD 54, 35–53 (2016)
P. Wu, G. Eriksson, A.D. Pelton, J. Am. Ceram. Soc. 76, 2059–2064 (1993)
M. Blander, A.D. Pelton, Geochim. Cosmochim. Acta 51, 85–95 (1987)
G. Eriksson, P. Wu, A.D. Pelton, CALPHAD 17, 189–205 (1993)
G. Eriksson, A.D. Pelton, Metall. Trans. B 24, 807–816 (1993)
B.C. Riggs, G.E. Plopper, J.L. Paluh, T.B. Phamduy, D.T. Corr, D.B. Chrisey, Proc. SPIE 8371, 83711F1-10 (2012)
Y. Harada, S. Sukenaga, N. Saito, K. Nakashima, ISIJ Int. 59, 1956–1965 (2019)
Y. Harada, N. Nishioka, N. Saito, K. Nakashima, ISIJ Int. 60, 42–50 (2020)
A.P. Sandoval, J.M. Feliu, R.M. Torresi, M.F. Suarez-Herrera, RSC Adv. 4, 3383–3391 (2014)
R.H. Fowler, E.A. Guggenheim, Statistical Thermodynamics (Cambridge University Press, Cambridge, 1939), pp. 350–366
H. Gaye, J. Welfringer, in Proceedings of the 2nd International Symposium on Metallurgical Slags and Fluxes. ed. by H.A. Fine, D.R. Gaskel (TMS-AIME, Warrendale, 1984), pp. 357–75
C. Le Losq, D.R. Neuville, J. Non-cryst. Solids 463, 175–188 (2017)
C. Spearman, Am. J. Psychol. 15, 72–101 (1904)
C.I. Merzbacher, B.L. Sherriff, J.S. Hartman, W.B. White, J. Non-cryst. Solids 124, 194–206 (1990)
H. Maekawa, T. Maekawa, K. Kawamura, T. Yokokawa, J. Non-cryst. Solids 127, 53–64 (1991)
T. Matsumiya, K. Shimoda, K. Saito, K. Kanehashi, W. Yamada, ISIJ Int. 47, 802–804 (2007)
T. Iida, Y. Kita, M. Ueda, K. Mori, K. Nakashima, Viscosity of Molten Slag and Glass (Agne Gijutsu Center, Tokyo, 2003), p. 77
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted July 24, 2020; accepted January 3, 2021.
Appendices
Appendix
(A) Calculation of Thermodynamic Parameters in Quasichemical Model
In this study, ternary molten oxide systems were assumed as quasi binary system consisting of NWF and NWM oxides. The calculations of the thermodynamic parameters of the quasichemical model in quasi binary systems, ω (the change in the enthalpy of the pair formation reaction) and η (the change in the non-configurational entropy of the pair formation reaction), are described below for the SiO2-Al2O3-CaO system as an example. In this case, the mole fraction of each oxide is represented by \(X_{{{\text{SiO}}_{2} }} ,\;X_{{{\text{Al}}_{2} {\text{O}}_{3} }} ,\) and XCaO.
First, the method for calculating NBO/T by the pair fraction of the system by “Equilib module” of FactSage is explained. The Equilib module of FactSage can calculate the bond information for bonds such as Si-Si-O-O. In the system of SiO2-Al2O3-CaO, the mole fractions of Si-Si-O-O, Si-Al-O-O, Si-Ca-O-O, Al-Al-O-O, Al-Ca-O-O, and Ca-Ca-O-O were obtained. Then, NBO/T was calculated considering that only the Si-Si-O-O bond was the bridging oxygen bond.
Second, the procedure for calculating the thermodynamic parameters ω and η is described below, and the 0.5SiO2-0.2Al2O3-0.3CaO system was separated by each binary system. In this case, Al2O3 was considered as AlO1.5: that is, 0.56SiO2-0.44Al2O3, 0.63SiO2-0.37CaO, and 0.57Al2O3-0.43CaO systems were considered. The thermodynamic parameters ω and η of each separated system were calculated with the help of values from the literature. In the case of the 0.56SiO2-0.44Al2O3 system, for example, when \(X^{\prime}_{{{\text{SiO}}_{2} }}\) was defined as the mole fraction of SiO2 (i.e., \(X^{\prime}_{{{\text{SiO}}_{2} }} = 0.56\)), thermodynamic parameters were given as below based on the literature.[32]
Similar calculations were performed to calculate the values of ωSi-Al, ωSi-Ca, ωAl-Ca, ηSi-Al, ηSi-Ca, and ηAl-Ca. From these parameters, the mixing enthalpy ΔH and the non-configurational entropy ΔSnc were calculated using the following equation:
where Rij (i, j = Si, Al, or Ca) is the pair fraction of [Mi − Mj] calculated by FactSage and bA (A= SiO2, Al2O3, or CaO) is called the equivalent fraction. The calculation was based on parameters such as \(b_{{{\text{SiO}}_{2} }} = 1.0331,\) where \(\left( {n/4} \right)b_{{{\text{SiO}}_{2} }}\) is given in the case of an n-valent cation, i.e., \(b_{{{\text{Al}}_{2} {\text{O}}_{3} }} = 1.0331\) and bCaO = 0.6887. The thermodynamic parameters of the quasi binary system were calculated using the values of ΔH and ΔSnc.
Then, the 0.5SiO2-0.2Al2O3-0.3CaO (mol pct) system was represented by quasi binary system composed of NWF and NWM oxides. In the case of the 0.5SiO2-0.2Al2O3-0.3CaO (mol pct) system, the amount of Al2O3 is larger than that of CaO. Therefore, it is assumed that Al2O3 was tetracoordinate. NWF and NWM were further defined as follows:
Furthermore, the pair fraction of RNWF–NWF, RNWF–NWM, and RNWM–NWM can be calculated by the pair fraction of the 0.5SiO2-0.2Al2O3-0.3CaO system estimated by FactSage. In particular, both Si and Al worked as NWF; therefore, the [Si-Al] pair, for example, was assumed as RNWF–NWF. Then, the following equations provided the thermodynamic parameters of a quasi binary system, ω and η, considering the values of pair fraction of the quasi binary system (RNWF–NWM), mole fraction of NWF and NWM (XNWF and XNWM), and ΔH and ΔSnc calculated using Eqs. [A1] and [A2].
(B) Calculation of the Pair Fraction of the Quasichemical Model
The calculation of the pair fraction of the quasi binary system was performed by using the thermodynamic parameters ω and η. For the composition mentioned in the above example, XNWF and XNWM were 0.9 and 0.1, respectively. In the quasichemical model, the pair fraction is expressed by the following equation:
where R and T are gas constant and absolute temperature, respectively. From the Eqs. [A5] and [A6], when (ω−ηT) is given, RNWF–NWM was calculated, the RNWF–NWF and RNWM–NWM were calculated by substituting RNWF–NWM for the following equations of mass conservation law:
The fraction of NBO and NBO/T were calculated by the estimated pair numbers.
Rights and permissions
About this article
Cite this article
Harada, Y., Saito, N. & Nakashima, K. Structural Evaluation of Silicate Melts by Performing Impedance Measurements and Quasichemical Model Calculations. Metall Mater Trans B 52, 968–977 (2021). https://doi.org/10.1007/s11663-021-02069-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02069-x