Abstract
Background
As breast cancer screening guidelines have changed recently, additional investigation is needed to understand changes in women’s behavior after using breast cancer screening patient decision aids (BCS-PtDAs) and the potential effect on mammography utilization. This systematic review and meta-analysis sought to evaluate the effect of BCS-PtDAs on changes in women’s intentions to undergo screening mammography and whether women deciding to begin or discontinue screening mammography displayed similar changes in screening intentions after using a BCS-PtDA.
Methods
We searched Medline, Scopus, PsycINFO, CENTRAL, Health and Psychosocial Instruments, Health Technology Assessment Database, PsycARTICLES, and cited references in eligible papers for randomized controlled trials (RCTs) and observational studies, published through August 24, 2016. The proportions of women who did and not intend to undergo screening and who were uncertain about undergoing screening mammography were pooled, using risk ratios (RR) and random effects. According to the protocol, RCTs or observational studies and any language were considered eligible for systematic review if they included data about women for which shared decision making is recommended.
Results
We ultimately included six studies with screening intention data for 2040 women. Compared to usual care, the use of BCS-PtDAs in three RCTs resulted in significantly more women deciding not to undergo screening mammography (RR 1.48 [95% CI 1.04–2.13]; P = 0.03), particularly for younger (38–50 years) women (1.77 [1.34-2.34]; P < 0.001). The use of BCS-PtDAs had a non-significant effect on the intentions of older women (69–89 years) to discontinue screening.
Conclusions
The use of BCS-PtDAs increased younger women’s reluctance to undergo screening for breast cancer. The implementation of such BCS-PtDAs in clinical practice would be expected to result in a 77% increase in the number of younger women (aged 38–50) who do not intend to be screened, and as a consequence, may reduce utilization of screening mammography.
Registration
The protocol of this review is registered in the PROSPERO database, #CRD42016036695.
Similar content being viewed by others
INTRODUCTION
According to the World Health Organization (WHO), breast cancer is the most common cancer among women in 140 countries and is the leading cause of cancer mortality in 101 countries.1 The American Cancer Society estimates2 that in 2017, approximately 252,710 women in the United States (U.S.) will be diagnosed with invasive breast cancer, and about 40,610 women will die from breast cancer. Because of the disease’s asymptomatic phase, mammography is recommended as a primary screening procedure for early diagnosis.3 However, it is expected that if 1000 U.S. women in their 40s with an average population risk for breast cancer undergo screening mammography today, then 125 of those women would be called back for additional testing.4 False-positive results elicit fear and distress in women and can lead to further imaging tests and/or biopsy. Criticism based on frequent false-positive findings, which occur at an even higher rate with annual screening than biennial screening,5 , 6 are reinforced by a lack of evidence that screening mammography significantly reduces breast cancer mortality among women in their 40s and 70s.7 Older women whose life expectancy is less than 10 years might not benefit from screening mammography.6 Another drawback of screening mammography is the high number needed to screen to prevent one death—in the case of biennial screening, equal to 1034 for women in their 40s, 426 for women in their 70s, and 1339 for women in their 80s.8 Additionally, a recent cohort study9 reported that screening mammography can result in overdiagnosis.
Due to the different interpretations of evidence around potential benefits and harms of screening mammography, current breast cancer screening recommendations are not uniform across countries10 – 12 and professional organizations (Fig. 1) including the WHO,3 American Cancer Society,6 United States Preventive Services Task Force,13 and Canadian Cancer Society.14 Nevertheless, recent recommendations3 , 6 , 12 , 13 do converge on the view that any decision regarding mammography screening for women at average risk of breast cancer should be based on age, knowledge of risks, personal values, and concerns (Fig. 1). Evidence-based patient decision aids (PtDAs)15 – 20 are currently utilized to facilitate shared decision making. The Cochrane Collaboration reports21 that PtDAs support shared decision making by increasing patients’ knowledge, enhancing the accuracy of risk perception, reducing patient decisional conflict, increasing patient satisfaction with a choice, and engaging patients in the decision-making process.
It is not known whether the implementation of the recommendations for shared decision making and the use of PtDAs in clinical practice at the national level will affect the rates of screening mammography utilization. The present paper seeks to understand the influence of breast cancer patient decision aids (BCS-PtDAs) on changes in women’s intentions to undergo screening mammography in age groups where shared decision making is recommended. The purpose of this systematic review was to answer the following key questions (KQs; Fig. 2):
-
KQ1: What effect do BCS-PtDAs have on changing younger and older women’s intentions to undergo screening mammography?
-
KQ2a: What effect do BCS-PtDAs have on changing younger women’s intentions to begin screening mammography (women in their 40s)?
-
KQ2b: What effect do BCS-PtDAs have on changing older women’s intentions to continue screening mammography (women in their 70s)?
METHODS
The protocol of this systematic review was registered in the PROSPERO database, #CRD42016036695.22
Eligibility Criteria, Information Sources, and Search Strategy
The present study employed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations for systematic reviews and meta-analyses.23 Studies were considered eligible for systematic review if they (i) reported data from primary studies; (ii) included data about women aged 38–50 and 69–89 who had not been diagnosed with breast cancer prior to using a PtDA; (iii) contained an intervention that was a PtDA; and (iv) were randomized controlled trials (RCTs), non-randomized studies, cohort studies, case–control studies, or before–after studies. The exclusion and inclusion criteria are presented in Online Appendix 1.
A search was conducted and updated using the following databases: Scopus (through August 24, 2016), MEDLINE Epub Ahead of Print (OvidSP), MEDLINE In-Process & Other Non-Indexed Citations (OvidSP), MEDLINE® Daily (OvidSP), and MEDLINE (OvidSP; 1946 to August 16, 2016), Cochrane Central Register of Controlled Trials [(CENTRAL) OvidSP; 1991 to July 2016], PsycINFO (OvidSP; 1806 to July [week 4] 2016), Health and Psychosocial Instruments (OvidSP; 1985 to July 2016), Health Technology Assessment (OvidSP; 2001 to third quarter 2016), PsycARTICLES Full Text (OvidSP; through August 16, 2016) (Online Appendix 2). In addition, we reviewed cited references in eligible studies.
Study Selection
All potentially eligible articles were independently screened by two reviewers (II, EH), who coded each article as “eligible,” “potentially eligible,” or “not eligible.” All papers classified as eligible with appropriate risk of evidence were included in the subsequent analysis. Articles ranked as “potentially eligible” or “not eligible” by at least one reviewer were examined again by both reviewers together and were then either classified as “eligible” or disqualified on the basis of consensus. If the two investigators could not reach consensus, an intermediary (KE) was consulted for final judgment. The same procedure was adopted for full-text papers of all potentially eligible studies. A list of included and excluded papers may be found in Online Appendix 3. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach24 was used to assess evidence quality.
Data Collection and Risk of Bias
Based on the Cochrane Consumers and Communication Review Group’s Data Extraction Template, a Microsoft Excel data extraction sheet was developed. The two reviewers (II, EH) extracted and assessed the following data for accuracy and completeness: author; year of publication; country; type of study; data collection period; number and age of patients; type of intervention; and proportion of women who were undecided, who intended to undergo mammography, and who did not wish to undergo mammography (Table 1). For RCTs, data were obtained for control and intervention groups. For before–after studies, data were abstracted pre/post-intervention. Two papers16 , 20 did not contain the requisite information about women’s screening intentions; the corresponding authors were contacted to obtain the required data in these cases. One study19 did not differentiate between the women who were unsure and the women who did not intend to undergo screening mammography. The corresponding author of that paper19 (Mara A. Schonberg, MD, MPH) was successfully contacted by email to identify the boundaries between groups of patients who were unsure and those who did not plan to continue with screening mammography. The results of the individual studies are presented in Table 1. The two investigators (II, EH) assessed the strengths and weaknesses of each eligible study. For studies that did not randomize patients, the National Institutes of Health Quality Assessment Tool for before–after (pre–post) studies with no control group was used.25 For randomized trials, the Cochrane Collaboration tool for assessing risk of bias was used.26
Synthesis of Results
Studies with an acceptable risk of bias (i.e., they did not meet more than three criteria) that were similar in clinical and methodological aspects were combined for meta-analysis. A random effects model27 was used to process the risk ratio (RR). Heterogeneity testing was conducted to establish the degree of inconsistency among studies and subgroups of studies, and an I 2 statistic was calculated.
The number of women who planned to undergo screening mammography was calculated as being equal to the total number of women minus the sum of unsure women and women who did not wish to undergo screening. The number of women who did not want to undergo screening mammography was defined as the total number of women minus the sum of women in the intended and undecided groups. The number of women who were undecided about undergoing screening mammography equaled the total number of women minus the sum of intended women and women who did not wish to undergo screening.
Neither funnel plots nor the Egger test28 was used to examine the effect of publication bias, since only six studies were ultimately included in the analysis.29 We did identify protocols for new or ongoing studies on the effects of BCS-PtDAs, however, and a future update of the current review will include more studies and will test for funnel plot asymmetry.
Sensitivity analyses were pre-specified. We examined the influence of (i) the chosen effects model, (ii) quasi-experimental studies, (iii) major outliers (i.e., those with the largest and lowest RR in analyses involving more than two studies were excluded in turn), and (iv) study quality and methodological diversity.
Data concerning women’s intentions were stored using Review Manager (RevMan) 5.3.530 software; the same software was used for the meta-analysis. For statistically significant results, we calculated the number required to be treated from the results of a meta-analysis of RR.31 The primary investigator (II) conducted the data synthesis, and its accuracy was verified by the collaborators (EH, KE, MM).
RESULTS
Study Selection
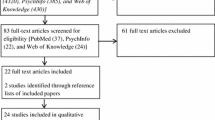
The search identified 422 potentially eligible records, of which six15 – 20 met the inclusion criteria (Online Appendix 1); 44 were duplicates, and 372 did not meet the inclusion criteria and thus were excluded (Fig. 3 and Online Appendix 3). Of the 372 excluded records, 363 were excluded after evaluation of the titles and abstracts. Nine articles were excluded after accessing full texts: two articles32 , 33 reported outcomes that were not relevant to the present review; two studies34 , 35 lacked any PtDA intervention; one article36 appeared in the wrong type of publication (study protocol); and four studies37 – 40 were based on unsuitable study designs.
Study Characteristics
Data were extracted from all six selected studies15 – 20 (Table 1). Of these, three were randomized controlled trials,15 , 17 , 18 while the other three were uncontrolled before–after studies.16 , 19 , 20 All six studies15 – 20 measured the proportion of women who (i) did not wish to start (aged 38–50)15 – 17 , 20 or continue (aged 69–89)18 , 19 screening mammography, (ii) intended to start (aged 38–50)15 – 17 , 20 or continue (aged 69–89)18 , 19 screening mammography, and (iii) were unsure about starting15 – 17 , 20 or continuing18 , 19 screening mammography. These data were available for all trials,15 , 17 , 18 which randomized 2025 patients and reported data regarding intention for 1869 women. Data on intention for 171 women from before–after studies16 , 19 , 20 were included; the meta-analysis included a total of 2040 women from six studies.
Breast Cancer Screening Patient Decision Aids
Three BCS-PtDAs16 , 17 , 20 were computerized and presented screening information as text and diagrams. Only one of these three16 used animation to provide information to women. The other three BCS-PtDAs were booklets15 , 18 or a pamphlet.19 The authors of four studies15 , 16 , 19 , 20 reported using International Patient Decision Aid Standards (IPDAS) when creating a BCS-PtDA; however, the level of IPDAS implementation differed among the BCS-PtDAs. Two BCS-PtDAs were developed using a decision support framework41 and were not guided by the IPDAS. Four BCS-PtDAs16 , 17 , 19 , 20 contained an algorithm that assessed the risk of breast cancer in users. Five of six BCS-PtDAs16 – 20 given to women described risk factors that could lead to breast cancer. Two BCS-PtDAs15 , 16 included an extensive list of medical terms that patients might be unfamiliar with; the other two BCS-PtDAs17 , 18 contained an explanation of one or two terms; one BCS-PtDA19 did not have an explanation of medical terms, and another BCS-PtDA20 was not available for this assessment. The level of personalization differed among BCS-PtDAs. Five BCS-PtDAs16 – 20 provided personalization, as they included interactive exercises to identify the patients’ values, critical factors for their decisions, and their expectations. All six BCS-PtDAs had some level of patient, caregiver, or stakeholder engagement in the development process. A detailed comparison of analyzed BCS-PtDAs is described in Online Appendix 4.
Risk of Bias Within Studies
The risk of bias was also assessed for each study, and all six studies were considered to have a low or moderate risk of bias (Online Appendix 5). The before–after studies recruited specific populations—for example, rural women16 and women who used social media20—thus limiting generalizability (the women in the articles were not typical of those among the general population who would be eligible for the intervention). The risk of performance bias for two RCTs17 , 18 was unclear. One study17 did not report on the allocation and did not provide a link to its protocol (Online Appendix 6).
Results of Individual Studies
Three studies17 – 19 examined the effect of PtDAs on women’s knowledge. All three studies reported a statistically significant improvement in knowledge after use of the BCS-PtDAs (P < 0.001). Five of the studies16 – 20 assessed the clarity of values; three papers15 , 17 , 18 used Dormandy’s multidimensional measure of informed choice42 to evaluate the clarity of values; two studies16 , 19 used values clarity subscale scores;43 and two studies16 , 18 indicated that the use of PtDAs significantly reduced women’s uncertainty about their values (P < 0.001 and P = 0.02). The other two studies17 , 19 were unable to identify statistically significant differences in clarity of values (P = 0.14 and P = 0.89). Two RCTs15 , 18 reported that a significantly greater number of women (P < 0.01) in the intervention group made an informed choice (choices were made among women with similar attitudes and values based on an adequate level of knowledge). Two before–after studies16 , 20 reported that women, after using a BCS-PtDAs, felt significantly more informed when making a decision on when they should begin screening mammography. One RCT17 and one before–after study19 did not indicate a significant change in the number of women who felt informed or were more likely to make an informed decision.
Synthesis of Results
Analysis of the overall effect of RCTs15 , 17 , 18 indicated that, compared to usual care interventions, BCS-PtDAs resulted in a statistically significant increase in the proportion of women (aged 38–50 and 69–71) who decided not to undergo screening following the use of a BCS-PtDA (RR 1.48; 95% CI 1.04–2.13; P = 0.03; n = 3; [I 2 = 54%; P = 0.11]; Fig. 4a, subgroup 1.1.1). The meta-analysis of the RCTs showed that in the usual care group, 105 of 1000 women decided not to be screened, compared to 155 (95% CI 109–223) of 1000 for the BCS-PtDA group. This suggests that an additional 50 women out of 1000 would not plan to be screened after using a BCS-PtDA (Table 2 and Online Appendix 7). Using a number-needed-to-treat approach, 20 women aged 38–50 and 69–71 (95% CI 9–239) would need to use a BCS-PtDA in order for one woman to decide not to undergo screening mammography. In contrast, the analysis of the before–after studies showed no statistically significant difference (RR 1.05; 95% CI 0.54–2.05; P = 0.89; Fig. 4a, subgroup 1.1.2). The three observational studies16 , 19 , 20 were all significantly smaller in size (one-way ANOVA F[1,4] = 13.57; P = 0.02), however, and were not powered to evaluate change in intention. The RCTs and before–after studies suggest that a higher proportion of women would not want to undergo screening mammography after using a BSC-PtDA (Table 2).
When different age categories were considered, the proportion of women in the younger age group (38–50) who decided not to begin screening increased (RR 1.77; 95% CI 1.34–2.34; P < 0.001; n = 2; [I 2 = 0%; P = 0.62] Fig. 4b, subgroup 1.2.1) after using a BCS-PtDA. Analysis of intentions among these women revealed that in the usual care group, 111/1000 would not plan to be screened, compared to 197/1000 (95% CI 149–261) for the PtDA group. This suggests that after using the BCS-PtDA, an additional 85/1000 women would change their initial plans and would decide not to begin screening (Table 2 and Online Appendix 8). This means that 12 women (95% CI 7–27) aged 38–50 years must use a BCS-PtDA for one woman to decide not to begin screening mammography. The before–after studies (Fig. 4b, subgroup 1.2.2) showed no change in intention after using the BSC-PtDA and were not evaluated with a number-needed screening approach.
For women aged 69–71 and 75–89, the RCT18 and observational study19 comparing women’s intentions to continue a screening program displayed a non-significant effect (Fig. 4c) of a BCS-PtDA, with no significant heterogeneity between studies (P = 0.24, I 2 = 28.9%). Among these women, the BCS-PtDAs appeared to have less effect on their willingness to discontinue screening (Table 2 and Online Appendix 9).
None of our analyses identified a statistically significant difference in the proportion of women who remained undecided, who had decided their screening plans, or who wanted to undergo screening mammography after using a BCS-PtDA (Online Appendix 10).
Exploration for Inconsistency and Results of Additional Analyses
The forest plot shown in Figure 4a visualizes any statistical inconsistency among women who did not plan to undergo screening mammography. Of the six studies, three15 , 17 , 18 used randomization and three16 , 19 , 20 did not. The direction of the effect across the studies is collectively consistent (with the exception of two studies16 , 20) and favors BCS-PtDAs over usual care for reducing intention to undergo screening. A random effects model was used to assess the impact of design differences on the overall result. The overall RR from the three RCTs was 1.48 (95% CI 1.04–2.13; P = 0.03; [I 2 = 54%; P = 0.11]); and the RR from the before–after studies was 1.05 (95% CI 0.54–2.05; P = 0.89; n = 3; [I 2 = 11%; P = 0.33]), although no difference among these subgroups was statistically significant (I 2 = 0%, P = 0.37). The difference between RCTs (women who decided not to undergo screening mammography) cannot be explained in terms of differences in the level of breast cancer risk or by differences in age or decision type (i.e., to begin or continue screening). The disparity in the pooled effect from RCTs and before–after studies (women aged 38–50 years who did not plan to undergo screening mammography) can be explained as the result of different study designs (I 2 = 75.5%; P = 0.04), and may be because the before–after studies16 , 20 included only women with an average risk of breast cancer. In these studies, the women were assessed for average risk before enrollment and again when using the BCS-PtDA. Initially, only a random effects model was used; the application of the fixed effects model did not alter any of the significant results that were obtained using the random effects model.
DISCUSSION
Summary of Evidence
The questions addressed by the present study were (i) whether BCS-PtDAs influence changes in women’s intentions to undergo screening mammography and (ii) whether women from different age groups, who face different screening decisions, display similar changes in screening intentions after using a BCS-PtDA. Our results indicate that the use of BCS-PtDAs increased reluctance to undergo screening, particularly in younger women (38–50 years). Additional analyses did not identify any effect of BCS-PtDAs on the proportion of women (aged 38–50 and 69–89 years) who planned to undergo screening mammography (Online Appendix 10). No evidence was found that BCS-PtDAs influenced the proportion of women who were unsure about starting screening or women who were unsure about discontinuing screening. This analysis of intention reveals that the implementation of BCS-PtDAs for women over the age of 68 years may not influence their plans to continue with screening mammography.
Limitations
We did not use any language restrictions in the search strategy, which was an advantage of the present study. However, the study has several limitations. First, the level of evidence varied across the studies that were included. Only three studies used controlled randomization; the other three were uncontrolled before–after studies with a low level of evidence. Second, all six of the included studies were performed in two countries (Australia and USA). Only three studies (one RCT15 and two before–after16 , 20) assessed for breast cancer risk and only enrolled women at average risk for breast cancer. Two of the RCTs17 , 18 and one before–after study19 included women who did not have a history of breast cancer but did not formally assess for risk of breast cancer. The use of a BCS-PtDA designed for average-risk women can mislead women who are at above-average risk for breast cancer and may result in inadequate preventive care. It is important to note that changes in women’s intentions to undergo breast cancer screening after using a PtDA can differ from actual patient behavior and should be tested in RCTs; however, there is evidence that screening intention correlates positively with actual patient screening behavior.45
Conclusions
As breast cancer screening guidelines have recently changed, additional investigation was necessary to fully understand the effect of breast cancer screening patient decision aids (BCS-PtDAs) on changes in women’s screening behavior in age groups for which shared decision making is recommended. To the best of our knowledge, this is the first comprehensive systematic review and meta-analysis of changes in women’s intentions to undergo mammography screening after using BSC-PtDAs. Our findings showed that BSC-PtDA use resulted in a significantly greater number of women—particularly younger women (38–50 years)—not planning to begin mammography screening than those under usual care. The analysis revealed that the implementation of BCS-PtDAs in clinical practice at the national level, in accordance with the new recommendations, may result in a 77% increase in the number of women aged 38–50 who would not want to begin mammography screening, compared to the corresponding number of women under usual care. The implementation of BCS-PtDAs for women over 68 years of age may not influence their plans to continue mammography screening. The results of this study should be considered by healthcare policymakers and managers when deciding whether to incorporate BCS-PtDAs into regular practice. We support the need for large-scale randomized controlled trials of evidence-based BCS-PtDAs and a subsequent update of our systematic review and meta-analysis, to better understand the behavior affected by BCS-PtDAs and possible change in utilization of screening mammography.
Abbreviations
- BCS-PtDA:
-
breast cancer screening patient decision aid
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- IPDAS:
-
International Patient Decision Aid Standards
- KQ:
-
key question
- PtDA:
-
patient decision aid
- RCT:
-
randomized controlled trial
- RR:
-
risk ratio
References
Stewart BW, Wild CP. Breast cancer. In: World Cancer Report 2014. Lyon: International Agency for Research on Cancer; 2014:362–373.
American Cancer Society. Breast Cancer. How Common Is Breast Cancer? http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics. Published 2016. Accessed 16 Feb 2017.
World Health Organization. WHO Position Paper on Mammography Screening. Geneva: World Health Organization; 2014:27–32.
Nelson HD, O’Meara ES, Kerlikowske K, Balch S, Miglioretti D. Factors associated with rates of false-positive and false-negative results from digital mammography screening: an analysis of registry data. Ann Intern Med. 2016;164(4):226. doi:10.7326/M15-0971.
Nelson HD, Pappas M, Cantor A, Griffin J, Daeges M, Humphrey L. Harms of breast cancer screening: systematic review to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):256–267. doi:10.7326/M15-0970.
Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk. JAMA. 2015;314(15):1599–1614. doi:10.1001/jama.2015.12783.
Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244. doi:10.7326/M15-0969.
Hendrick RE, Helvie MA. Mammography screening: a new estimate of number needed to screen to prevent one breast cancer death. Am J Roentgenol. 2012;198(3):723–728. doi:10.2214/AJR.11.7146.
Jørgensen K, Gøtzsche PC, Kalager M, Zahl P. Breast cancer screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017. doi:10.7326/M16-0270.
Li J, Shao Z. Mammography screening in less developed countries. Springerplus. 2015;4(1):615. doi:10.1186/s40064-015-1394-8.
Biesheuvel C, Weige S, Heindel W. Mammography screening: evidence, history and current practice in Germany and other European countries. Breast Care. 2011;6(2):104–109. doi:10.1159/000327493.
Australian Government Department of Health. About breast screening. BreastScreen Australia Program. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/about-breast-screening. Published March 2015. Accessed 16 Feb 2017.
Siu AL. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279. doi:10.7326/M15-2886.
Canadian Cancer Society. Screening for breast cancer. Screening mammography. http://www.cancer.ca/en/cancer-information/cancer-type/breast/screening/?region=on. Accessed 16 Feb 2017.
Hersch J, Barratt A, Jansen J, et al. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: a randomised controlled trial. Lancet. 2015;385(9978):1642–1652. doi:10.1016/S0140-6736(15)60123-4.
Eden KB, Scariati P, Klein K, et al. Mammography decision aid reduces decisional conflict for women in their forties considering screening. J Women’s Health. 2015;24(12):1013–1020. doi:10.1089/jwh.2015.5256.
Mathieu E, Barratt AL, McGeechan K, et al. Helping women make choices about mammography screening: an online randomized trial of a decision aid for 40-year-old women. Patient Educ Couns. 2010;81(1):63–72. doi:10.1016/j.pec.2010.01.001.
Mathieu E, Barratt A, Davey HM, McGeechan K, Howard K, Houssami N. Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women. Arch Intern Med. 2007;167(19):2039–2046. doi:10.1001/archinte.167.19.2039.
Schonberg MA, Hamel MB, Davis RB, et al. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Intern Med. 2014;174(3):417. doi:10.1001/jamainternmed.2013.13639.
Scariati P, Nelson L, Watson L, Bedrick S, Eden KB. Impact of a decision aid on reducing uncertainty: pilot study of women in their 40s and screening mammography. BMC Med Inform Decis Mak. 2015;15(1):89. doi:10.1186/s12911-015-0210-2.
Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. In: Stacey D, ed. Cochrane Database of Systematic Reviews. Vol 1. Chichester, UK: John Wiley & Sons, Ltd; 2014:CD001431. doi:10.1002/14651858.CD001431.pub4.
Ivlev I, Hickman EN, McDonagh MS, Eden KB. Women’s change in intention to undergo screening mammography after using a patient decision aid: a systematic review and meta-analysis. 2016. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016036695. Accessed 16 Feb 2017.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7), e1000097. doi:10.1371/journal.pmed.1000097.
Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi:10.1016/j.jclinepi.2010.04.026.
National Institutes of Health. Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after. Published April 2014. Accessed 16 Feb 2017.
The Cochrane Collaboration. Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Altman D, Sterne J, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration; 2011. Available at: www.cochranehandbook.org. Accessed 16 Feb 2017.
Harris RJ, Bradburn MJ, Deeks JJ, Altman DG, Harbord RM, Sterne JAC. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8(1):3–28.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629.
Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi:10.1136/bmj.d4002.
Review Manager (RevMan) [Computer program]. Version 5.3.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
The Cochrane Collaboration. Computing absolute risk reduction or NNT from a risk ratio (RR). In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; 2011. Available at: www.cochranehandbook.org. Accessed 1 June 2016.
Lewis CL, Pignone MP, Sheridan SL, Downs SM, Kinsinger LS. A randomized trial of three videos that differ in the framing of information about mammography in women 40 to 49 years old. J Gen Intern Med. 2003;18(11):875–883. doi:10.1046/j.1525-1497.2003.21152.x.
Pasternack I, Saalasti-Koskinen U, Mäkelä M. Decision aid for women considering breast cancer screening. Int J Technol Assess Health Care. 2011;27(4):357–362. doi:10.1017/S026646231100050X.
Lewis CL, Kistler CE, Amick HR, et al. Older adults’ attitudes about continuing cancer screening later in life: a pilot study interviewing residents of two continuing care communities. BMC Geriatr. 2006;6(1):10. doi:10.1186/1471-2318-6-10.
Nojomi M, Namiranian N, Myers RE, Razavi-Ratki S-KK, Alborzi F. Factors associated with breast cancer screening decision stage among Women in Tehran, Iran. Int J Prev Med. 2014;5(2):196–202.
Hersch J, Barratt A, Jansen J, et al. The effect of information about overdetection of breast cancer on women’s decision-making about mammography screening: study protocol for a randomised controlled trial. BMJ Open. 2014;4(5), e004990. doi:10.1136/bmjopen-2014-004990.
Lin JW, Chu PL, Liou JM, Hwang JJ. Applying a multiple screening program aided by a guideline-driven computerized decision support system - A pilot experience in Yun-Lin, Taiwan. J Formos Med Assoc. 2007;106(1):58–68. doi:10.1016/S0929-6646(09)60217-5.
Mazurowski MA, Zurada JM, Tourassi GD. Selection of examples in case-based computer-aided decision systems. Phys Med Biol. 2008;53(21):6079–6096. doi:10.1088/0031-9155/53/21/013.
Povyakalo AA, Alberdi E, Strigini L, Ayton P. How to discriminate between computer-aided and computer-hindered decisions: a case study in mammography. Med Decis Mak. 2013;33(1):98–107. doi:10.1177/0272989X12465490.
Tisnado DM, Moore AA, Levin JR, Rosen S. Developing and testing a decision aid for use by providers in making recommendations: about mammography screening in older women. J Appl Gerontol. 2015;34(3):343–358. doi:10.1177/0733464812467397.
O’Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33(3):267–279. doi:10.1016/S0738-3991(98)00026-3.
Dormandy E, Michie S, Hooper R, Marteau TM. Informed choice in antenatal Down syndrome screening: a cluster-randomised trial of combined versus separate visit testing. Patient Educ Couns. 2006;61(1):56–64. doi:10.1016/j.pec.2005.02.006.
O’Connor AM. Validation of a decisional conflict scale. Med Decis Mak. 1995;15(1):25–30. doi:10.1177/0272989X9501500105.
Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables - binary outcomes. J Clin Epidemiol. 2013;66(2):158–172. doi:10.1016/j.jclinepi.2012.01.012.
Harada K, Lee S, Shimada H, et al. Psychological predictors of participation in screening for cognitive impairment among community-dwelling older adults. Geriatr Gerontol Int. 2016. doi:10.1111/ggi.12841.
Acknowledgements
This study was supported by United States National Library of Medicine Biomedical Informatics Training grant no. T15LM007088. The grantor had no role in the design or conduct of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Andrew Hamilton (Oregon Health & Science University) for help with the search strategy.
Contributors
II came up with the research idea. II, EH, MM, and KE designed the protocol. II and EH were responsible for data collection, extraction, and assessment of risk of bias. Statistical supervision was provided by II, and input by KE and MM. II wrote the first draft of the report and collated subsequent inputs. All authors commented and edited the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2366 kb)
Rights and permissions
About this article
Cite this article
Ivlev, I., Hickman, E.N., McDonagh, M.S. et al. Use of patient decision aids increased younger women’s reluctance to begin screening mammography: a systematic review and meta-analysis. J GEN INTERN MED 32, 803–812 (2017). https://doi.org/10.1007/s11606-017-4027-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-017-4027-9