ABSTRACT
BACKGROUND
Cardiovascular disease (CVD) prevention in diabetes requires broad-based treatment of dyslipidemia, hypertension, and hyperglycemia. The independent contribution of all combinations of risk factor control to CVD risk has not been evaluated.
OBJECTIVE
To estimate the independent association of control of glycosylated hemoglobin (A1C), systolic blood pressure (SBP), and low-density lipoprotein cholesterol (LDL-C) with risk of cardiovascular disease hospitalization.
DESIGN
Non-concurrent longitudinal cohort study.
PATIENTS
The study included 26,636 patients with type 2 diabetes who were members of an integrated group model HMO with multiple A1C, SBP, and LDL-C measurements.
MAIN MEASURES
Patients were followed for a mean (SD) of 5.6 (2.5) years until they died or disenrolled, or until 31 December 2010. The outcome was a first-observed CVD hospitalization. Using the mean of all A1C, SBP, and LDL-C measures during follow-up, we created dichotomous categories of A1C control (< 7 %), SBP control (< 130 mmHg), and LDL-C control (< 100 mg/dL) to estimate the incidence rate of CVD hospitalization associated with all combinations of risk factor control adjusting for demographic and clinical characteristics.
KEY RESULTS
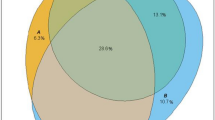
Patients with no controlled risk factors (18.2/1,000 person-years, 95 % CI 16.5−20.2) or with only A1C in control (16.9, 15.0−19.0) had the highest rate of CVD hospitalization, whereas those with all three risk factors controlled (7.2, 6.2−8.4) or with SBP and LDL-C in control (6.1, 5.1−7.2) had the lowest rates. Those with only SBP or LDL-C in control, A1C and SBP controlled, or A1C and LDL-C controlled had statistically similar incidence between the highest and lowest rates.
CONCLUSIONS
Maintaining SBP < 130 mmHg or LDL-C < 100 mg/dL was significantly associated with reduced CVD hospitalization risk, especially when both risk factors were well controlled. Maintaining A1C < 7 % was not independently associated with reduced CVD hospitalization risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Management of type 2 diabetes requires a multifaceted approach to optimize control of metabolic risk factors.1 Despite recent evidence that tight glycemic control does not appear to reduce cardiovascular disease (CVD) risk,2–4 epidemiologic analyses have consistently shown an association between higher glycosylated hemoglobin (A1C) and poor CVD outcomes.5–7 In addition, evidence-based guidelines recommend more aggressive therapeutic targets for the treatment of blood pressure and lipids in patients with diabetes than are suggested for the general population.8,9 Evidence behind these recommendations comes from clinical trials that studied treatment of specific risk factors without simultaneously intervening on other risk factors. One exception was the Steno-2 Study, which found that intensive intervention with multiple drug combinations was associated with significantly lower risk of CVD events, although no attempt was made to ascertain the independent contribution of each treated risk factor to CVD risk reduction.10
Preventing CVD in diabetes requires broad-based treatment of dyslipidemia, hypertension, and hyperglycemia.11,12 Other observational studies have examined the additive effects of glycemia and blood pressure and glycemia and dyslipidemia.13,14 To our knowledge, however, no study has simultaneously evaluated the independent contribution of all three of these risk factors to CVD risk. Therefore, our objective was to study the CVD benefits of control of A1C, systolic blood pressure (SBP), and low-density lipoprotein cholesterol (LDL-C), alone and in all combinations, as predictors of CVD hospitalization in a large cohort of patients with type 2 diabetes.
METHODS
Kaiser Permanente Northwest (KPNW) is an integrated healthcare delivery system that provides comprehensive medical services to approximately 480,000 individuals in a 75-mile radius around Portland, Oregon. An electronic medical record has been in use since 1996 that links encounter diagnoses, laboratory results, and pharmaceutical dispensings. The present non-concurrent longitudinal cohort study was approved by the KPNW Institutional Review Board with a waiver of informed consent.
Sample Selection
Since 1989, KPNW has maintained a diabetes registry that identifies members with diabetes from pharmacy, laboratory and encounter databases. Patients enter the registry on the basis of anti-hyperglycemic dispenses, diagnostic-level fasting glucose or A1C values, and inpatient or outpatient diagnoses (ICD-9-CM 250.xx). Clinicians remove patients from the registry who they believe have been entered erroneously. We identified all patients who entered the registry in 2007 or earlier and who had an eligibility period between 2002 and 2010 (n = 53,250). To ensure we were studying patients with type 2 diabetes, we excluded 5,514 individuals with an insulin dispense within the first year of diabetes recognition. Patients under age 18 years (n = 378) were also excluded. All patients were required to have A1C, LDL-C, and SBP measured after diabetes diagnosis but no more than 6 months apart, resulting in the exclusion of 12,681 patients. The first occurrence of the three-test combination was used as the baseline set of measurements, and the latest date that one of the baseline measurements was recorded was defined as the index date. We excluded 4,042 individuals who had a CVD hospitalization prior to the index date. Last, all subjects were required to have at least one additional A1C, SBP and LDL-C measurement during follow-up, resulting in a final sample size of 26,636 patients.
Outcome, Observation Period, and Exposure Variables
Using the electronic medical record, we followed patients from the index date until a hospital admission was recorded with a primary diagnosis of coronary heart disease (ICD-9-CM codes 410.x, 411.x, 413.x, 414.x) or stroke (430.x, 431.x, 432.x, 434.x, 435.x, 436.x, 437.1), defining the composite as CVD. Patients were followed from index date until they first experienced the outcome, died or left the health plan, or until 31 December 2010.
We used the mean of all available measures of A1C, SBP, and LDL-C during the observation period to examine the association between these risk factors and CVD hospitalizations. We analyzed each risk factor continuously and as dichotomous variables, using guideline-recommended levels of control (A1C < 7 %, SBP < 130 mmHg, LDL-C < 100 mg/dL). In addition, we created eight categories representing all possible combinations of risk factor control: 1) none of the three risk factors controlled; 2) only A1C controlled; 3) only SBP controlled; 4) only LDL-C controlled; 5) A1C and SBP controlled, but not LDL-C; 6) A1C and LDL-C controlled, but not SBP; 7) SBP and LDL-C controlled, but not A1C; and 8) A1C, SBP, and LDL-C all controlled.
Covariates
Covariates included baseline age sex, race, and duration of diabetes (defined as the time between entry into the diabetes registry and the index date). Although we excluded patients with a previous CVD hospitalization, some patients had CVD diagnosed in the outpatient setting during observation. Therefore, we included a covariate for outpatient-diagnosed CVD (same ICD-9 codes as for the outcome), as well as the following comorbidities: heart failure (ICD-9 428.x), retinopathy (250.5, 369.x, 362.01-362.07), neuropathy (250.6, 358.1, 713.5, 337.1, 357.2), depression (296.2, 296.3, 400.4, 309.1, 311), and chronic kidney disease (GFR <60 mL/min/1.73 m2, estimated from serum creatinine using the Modification of Diet in Renal Disease (MDRD) equation15). We also controlled for use of specific antihyperglycemic agents (metformin, sulphonylureas, thiazolidinediones, insulin, other agents), antihypertensive agents (angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARBs], β-blockers, other agents), antilipidemic agents (statins, fibrates, other agents), and antidepressants used within 100 days of the event (or the end of observation).
Statistical Analyses
We compared demographic and clinical characteristics, comorbidities, and pharmacotherapies among patients who did and did not experience a CVD event using t-tests for continuous variables and χ 2 tests for categorical variables. P values < 0.05 were considered significant. We also compared A1C, SBP, and LDL-C among patients who did and did not experience a CVD event using t-tests for the continuous values and χ 2 tests for the dichotomous indicators of risk factor control and for the distribution of all possible combinations of risk factor control.
We calculated incidence rates for CVD hospitalizations per 1,000 person-years for each of the possible combinations of risk factor control adjusted for age, sex and diabetes duration, using generalized linear regression with Poisson errors and the natural log of person-years as an adjustment for unequal follow-up using Proc Genmod in SAS v9.2 (SAS Institute, Cary, NC). A p value of 0.05 was used to calculate 95 % confidence intervals. We used Cox proportional hazards regression analysis to further adjust for clinical characteristics, comorbidities, and pharmacotherapy variables described above. The first Cox model used continuous measures of A1C, SBP, and LDL-C, a second used non-mutually exclusive dichotomous variables of risk factor control, and the final regression model included all possible combinations of risk factor control, using “All Three Risk Factors in Control” as the reference group. We tested the proportional hazards assumption by including time-dependent variables for all combinations of risk factor control in a Cox model; none were significant at p < 0.05, satisfying the assumption.
Sensitivity Analyses
We conducted three sensitivity analyses to confirm our findings. First, we used baseline measures of A1C, SBP, and LDL-C to analyze their association with risk of CVD hospitalization. Second, we substituted the last A1C, SBP, or LDL-C measurement prior to the event (or end of follow-up) for mean values and re-estimated the Cox models. Third, we repeated our analysis using mean values excluding individuals with mean A1C values < 6 %.
RESULTS
Of the 26,636 study patients, 1,943 (7.3 %) experienced a CVD hospitalization during the observation period (Table 1). Patients who experienced the outcome were older, more likely to be men, and had longer diabetes duration compared with those who remained event-free. All comorbidities except depression were more common among patients who experienced a CVD hospitalization as were use of several medications.
Mean A1C during follow-up did not differ between those who did and did not experience a CVD hospitalization (Table 2). However, mean SBP and LDL-C were significantly greater among those who experienced an event. Similarly, the proportion of patients with A1C in control was not significantly different, but the proportion with SBP control and LDL-C control was significantly lower among those who experienced an event.
Figure 1 displays CVD hospitalization incidence rates per 1,000 person-years for each of the eight mutually exclusive categories of A1C, SBP, and LDL-C control, adjusted for age, sex, and duration of diabetes. Patients with no controlled risk factors or with only A1C controlled had the highest CVD hospitalization rates, whereas those with all three risk factors controlled or with SBP and LDL-C controlled had the lowest rates. Patients with only SBP controlled, only LDL-C controlled, A1C and SBP controlled, or A1C and LDL-C controlled had statistically similar incidence rates that were significantly lower than those with no risk factors or only A1C controlled, and significantly higher than those with SBP and LDL-C or all three risk factors controlled.
After adjustment for demographic and clinical characteristics, comorbidities and pharmacotherapies, an increase of one standard deviation of SBP was associated with a 40 % increased risk of CVD hospitalization, as was one standard deviation increase of LDL-C (Table 3). Mean A1C was not associated with CVD risk. When A1C control, SBP control, and LDL-C control were included in models as non-mutually exclusive variables, SBP and LDL-C control were protective while A1C control was associated with a 14 % increased risk of CVD hospitalization. In adjusted models using all possible combinations of risk factor control, all categories, except having both SBP and LDL-C controlled, produced statistically significant adjusted hazard ratios relative to those with all three risk factors controlled.
Results of the sensitivity analyses are shown in the online appendix, supplementary Table 1. Associations between baseline measures of A1C, SBP, and LDL-C and CVD hospitalization were substantially weaker than mean values during observation. Substituting last values observed during follow-up produced results similar to those obtained using mean values. Exclusion of patients with mean A1C < 6 % attenuated the risk associated with A1C control, but did not change the results. Supplementary Table 2 in the online appendix shows the mean risk factor levels for the eight mutually exclusive categories. Similar values were seen among categories that indicated a specific risk factor was controlled and also among categories that indicated a specific risk factor was not controlled.
DISCUSSION
Clinical management of type 2 diabetes includes control of glycemia, blood pressure, and LDL-C to reduce the risk of CVD and other complications.1 In the current observational study of 26,636 patients with type 2 diabetes followed over a mean of approximately 6 years, we found that controlling all three risk factors was associated with an incidence rate of CVD hospitalization that was approximately 2.5 times lower than if none of the risk factors was below guideline-recommended levels.
Despite guidelines recommending multi-factorial treatment of cardiometabolic risk factors, there are surprisingly few studies that have examined the simultaneous benefits of risk factor control. The ADVANCE study reported that combined treatment of A1C and SBP (compared with no active intervention) had no effect on the incidence of macrovascular events, but lipid control was not included.16 A large observational study found that tight control of A1C and SBP reduced CVD risk by 33 %, but did not attempt to disentangle the relative benefits of the two risk factors, nor did it include lipid control as an analysis variable.17 The UKPDS explored the additive effects of glycemia and blood pressure,13 and a report from the Swedish National Diabetes Register analyzed the additive effects of glycemia and dyslipidemia.14 To our knowledge, the only study to evaluate all three cardiometabolic risk factors simultaneously was the Steno-2 study, which demonstrated that intensive treatment of A1C, SBP, and lipids reduced the risk of cardiovascular events by 53 %10; an effect that was sustained well after the intervention ceased.18 The Steno-2 sample size (n = 160) was too small to determine which risk factor or combination of risk factors accounted for the effect. To our knowledge, the current study is the first to simultaneously evaluate the contribution of all possible combinations of A1C, SBP, and LDL-C control to CVD risk reduction.
Maintaining A1C control below 7 % was not associated with reduced CVD hospitalization risk below that obtained with SBP and LDL-C control. CVD hospitalization incidence per 1,000 person-years among those with A1C in control but neither of the other risk factors in control was statistically similar to incidence found among those with no risk factors in control; incidence among those with A1C in control in addition to either SBP or LDL-C was statistically similar to the rate among those with only SBP or LDL-C in control, respectively; and control of both SBP and LDL-C, but not A1C, produced a statistically similar rate to that of patients who had all three risk factors controlled. Our findings will likely contribute to the emerging controversy over optimal A1C levels. Glycemic control remains a cornerstone of good diabetes care. Despite clinical trials that found no CVD benefit and possible harm with tight control,2–4 an A1C level < 7 % is still considered optimal for most patients,12 although a less stringent patient-centered approach has recently been recommended.19 The recommendation to achieve A1C < 7 % was initially due to UKPDS evidence that intensive control substantially reduced microvascular complications,20 and more recent clinical trials re-affirm the microvascular benefit of low A1C levels.3,21 The nature of the relationship between A1C and CVD risk, however, remains unclear. Over a decade ago, the UKPDS found that each 1 % reduction in A1C was associated with a 14 % reduced risk of myocardial infarction, with no threshold below which risk reduction could not be obtained.5 More recent observational studies conducted on large samples in Sweden and New Zealand reported similar findings.22,23 However, two other studies found a U-shaped relationship, with levels of A1C < 6%−6.5 % and > 8.5 % conferring risk of cardiovascular outcomes or death.6,24 A post-hoc analysis from the ADVANCE trial found evidence of a threshold such that A1C < 7 % did not reduce macrovascular events.25 The current study did not find an association between mean A1C < 7 % and reduced CVD risk after controlling for other risk factors. One possible explanation is that 90 % of patients had mean A1C levels < 9 %; the benefits of additional glycemic control in a relatively well-controlled sample may be difficult to detect. Our A1C results may also be confounded by non-random use of anti-hyperglycemic agents, specifically metformin and sulfonylureas, that are known to have differential CVD effects.26,27
All categories of risk factor control that included SBP were associated with lower CVD hospitalization incidence than similar categories that did not. For example, CVD hospitalization incidence among those with only SBP in control was lower than among those with no risk factors in control, and incidence among those with SBP and LDL-C in control was lower than among those with only LDL-C in control. Our definition of SBP control (< 130 mmHg) was based on the American Diabetes Association guidelines that were in place during the observation period.1 However, this level of SBP control is somewhat controversial. The American College of Cardiology/American Heart Association, for example, holds that < 140 mmHg is sufficient,28 a level supported by recent trials. The ACCORD trial found that targeting SBP < 120 mmHg, as compared with < 140 mmHg, did not reduce the rate of major cardiovascular events.29 Another recent study also suggested that tight SBP control (< 130 mmHg) was not associated with improved CVD outcomes relative to usual control of 130−139 mmHg, but did provide substantial benefit compared with SBP ≥ 140 mmHg.30 The Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) found no benefit in fatal or nonfatal cardiovascular outcomes by reducing SBP below 130 mmHg.31 Nevertheless, our results suggest that achieving and maintaining SBP at that level is associated with CVD risk reduction.
Similar to SBP control, we found that all categories of risk factor control that included LDL-C were associated with lower CVD hospitalization incidence than similar categories that did not. CVD hospitalization incidence among those with only LDL-C in control was lower than among those with no risk factors in control, and CVD hospitalization incidence among those with SBP and LDL-C in control was lower than among those with only SBP in control. Because diabetes may be a cardiovascular risk equivalent,32 current guidelines recommend an LDL-C target of < 100 mg/dL,8 or perhaps as low as < 70 mg/dL, for patients with diabetes.33 We did not test categories of control other than < 100 mg/dL, so we cannot determine whether a “floor” for LDL-C control exists.
Strengths of the study include its large sample size and the designed intent to study all possible combinations of control of three key cardiometabolic risk factors. There are several limitations. We required all three risk factors to be measured within 6 months of each other, resulting in the exclusion of 27 % of the eligible sample. However, excluded patients did not differ demographically from the study sample. As an observational study, we cannot conclude that the reported associations between risk factor control and CVD hospitalization risk are causal. Although we controlled for characteristics and risk factors that might affect CVD, residual confounding may exist. We included covariates for medications and duration of diabetes, but did not explore the myriad interactions between specific medications, dosages, and duration of therapy. We used death as a censoring event and some deaths could have occurred from cardiovascular causes outside the hospital, resulting in an underestimate of CVD rates. Moreover, A1C control may affect mortality differently than CVD hospitalizations.2,24 We did not attempt to adjudicate CVD hospitalizations, relying on the accuracy of coding of inpatient diagnoses.
In summary, we found that maintaining SBP < 130 mmHg or LDL-C < 100 mg/dL over a mean follow-up of approximately 6 years was significantly associated with reduced risk of CVD hospitalization. The effect was especially strong when both of these risk factors were well controlled. Maintaining A1C < 7 % in an already well-controlled population was not associated with CVD hospitalization risk reduction.
REFERENCES
American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(Suppl 1):S11–S63.
Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139.
Stratton IM, Alder AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412.
Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case–control study. Diabetes Care. 2011;34(1):77–83.
Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811.
Grundy SM, Cleeman JI, Bairey-Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239.
National High Blood Pressure Education Program Working Group. National High Blood Pressure Education Working Group report on hypertension in diabetes. Hypertension. 1994;23(2):145–158.
Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393.
Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30(1):162–172.
Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EAM, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA Diabetes trials. Diabetes Care. 2009;32(1):187–192.
Stratton IM, Matthews DR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia. 2006;49:1761–1769.
Gudbjornsdotir S, Eliasson B, Eeg-Olofsson K, Zethelius B, Cederholm J. on behalf of the National Diabetes Register (NDR). Additive effoects of glycaemia and dyslipidemia on risk of cardiovascular disease in type 2 diabetes: an observational study from the Swedish National Diabetes Register. Diabetologia. 2011;54:2544–2551.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470.
Zoungas S, de Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: New results from the ADVANCE trial. Diabetes Care. 2009;32(11):2068–2074.
Cederholm J, Zethelius B, Nilsson PM, Eeg-Olofsson K, Eliasson B, Gudbjornsdottir S, et al. Effect of tight control of HbA1c and blood pressure on cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diab Res Clin Pract. 2009;86:74–81.
Gaede P, Lund-Anderson H, Parving HH, Pederson O. Effect of a multi-factorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of Hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care. 2012;35(6):1364–1379.
UKPDS Group. UKPDS 33: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with Type 2 diabetes. Lancet. 1998;352:837–851.
Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430.
Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdotir S, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study for the Swedish National Diabetes Register (NDR). J Intern Med. 2010;268(5):471–482.
Elley CR, Kenealy T, Robinson E, Drury PL. Glycated haemoglobin and cardiovascular outcomes in people with type 2 diabetes: a large prospective cohort study. Diabet Med. 2008;25:1295–1301.
Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–489.
Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55(3):636–643.
Jorgensen CH, Gislason GH, Bretler D, Sorensen R, Norgaard ML, Hansen ML, et al. Glyburide increases risk in patients with diabetes mellitus after emergent percutaneous intervention for myocardial infarction—A nationwide study. Int J Cardiol. 2011;152(3):327–331.
Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, Marinopoulos SS, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008;168(19):2070–2080.
Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2011;57(20):2037-114.
Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585.
Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304(1):61–68.
Redon J, Mancia G, Sleight P, Schumacher H, Gao P, Pogue J, et al. Safety and efficacy of low blood pressures among patients with diabetes: subgroup analyses from the ONTARGET (ONgoing telmisartan alone and in combination with ramipril global endpoint trial). J Am Coll Cardiol. 2012;59(1):74–83.
Schramm TK, Gislason GH, Kober L, Rasmussen S, Rasmussen JN, Abildstrom SZ, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945–1954.
Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512–1524.
Conflicts of Interest
Funding for this research was provided by AstraZeneca LP and Bristol Myers-Squibb, and two of the coauthors are employees and shareholders of AstraZeneca. As coauthors, they contributed to the design and reporting of the study. By contract, however, the lead author (GAN), who is not affiliated with AstraZeneca or Bristol Myers-Squibb, had control of the data and retained final authority over design, content, and interpretation of the analyses. These data were presented in poster form at the 72nd Scientific Sessions of the American Diabetes Association in June, 2012.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1.
Sensitivity Analyses of Adjusted Hazard Ratios for Continuous Measures of Risk Factors, Dichotomous Measures of Risk Factor Control, and All Possible Combinations of Risk Factor Control (DOC 58 kb)
Supplemental Table 2.
Mean Values (SD) of A1C, Systolic Blood Pressure and LDL Cholesterol by Mutually Exclusive Categories of Control (DOC 32 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nichols, G.A., Joshua-Gotlib, S. & Parasuraman, S. Independent Contribution of A1C, Systolic Blood Pressure, and LDL Cholesterol Control to Risk of Cardiovascular Disease Hospitalizations in Type 2 Diabetes: An Observational Cohort Study. J GEN INTERN MED 28, 691–697 (2013). https://doi.org/10.1007/s11606-012-2320-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-012-2320-1