Abstract
Background
The purpose of this study was to develop and validate a prediction model and clinical risk score for Intensive Care Resource Utilization after colon cancer surgery.
Methods
Adult (≥ 18 years old) patients from the 2012 to 2018 ACS-NSQIP colectomy-targeted database who underwent elective colon cancer surgery were identified. A prediction model for 30-day postoperative Intensive Care Resource Utilization was developed and transformed into a clinical risk score based on the regression coefficients. Model performance was assessed using the area under the receiver operating characteristic curve (AUC) and Hosmer-Lemeshow goodness-of-fit test. The model was validated in a separate test set of similar patients.
Results
In total, 54,893 patients underwent an elective colon cancer resection, of which 1224 (2.2%) required postoperative Intensive Care Resource Utilization. The final prediction model retained six variables: age (≥ 70; OR 1.90, 95% CI 1.68–2.14), sex (male; OR 1.73, 95% CI 1.54–1.95), American Society of Anesthesiologists score (III/IV; OR 2.52, 95% CI 2.15–2.95), cardiorespiratory disease (yes; OR 2.22, 95% CI 1.94–2.53), functional status (dependent; OR 2.81, 95% CI 2.22–3.56), and operative approach (open surgery; OR 1.70, 95% CI 1.51–1.93). The model demonstrated good discrimination (AUC = 0.73). A clinical risk score was developed, and the risk of requiring postoperative Intensive Care Resource Utilization ranged from 0.03 (0 points) to 19.0% (8 points). The model performed well on test set validation (AUC = 0.73).
Conclusion
A prediction model and clinical risk score for postoperative Intensive Care Resource Utilization after colon cancer surgery was developed and validated.
Similar content being viewed by others
Introduction
Colon cancer is the second most common cause of cancer-related mortality in the USA [1], and it is anticipated that there will 147,950 new cases diagnosed in the year 2020 [2]. While surgical resection is the mainstay of curative therapy, the COVID-19 pandemic has greatly impacted the delivery of care to colon cancer patients. As the number of new cases of SARS-CoV-2 infection continues to grow across North America, elective surgeries have come to a halt, with cancer cases presenting a unique and difficult challenge [3]. Hospital administrators are faced with the task of balancing the risk of SARS-CoV-2 infection and healthcare resource utilization with that of disease progression associated with treatment delays [4]. In the management of colon cancer, this task becomes especially difficult, as no accepted treatment alternative exists for most cases. Colon cancer surgery is thus considered a medically necessary and time-sensitive procedure, even during the COVID-19 pandemic [5], and societal and provincial guidelines have prioritized these cases among the most urgent of procedures.
Among all hospital resources, intensive care unit (ICU) beds, equipment, and personnel are perhaps the most precious in the current healthcare environment. Based on data from Italy and China, it is estimated that approximately 15% of patients presenting with SARS-CoV-2 will require ICU admission [6, 7], and ventilator shortages have been of paramount concern. Surgical patients are typically a large consumer of Intensive Care Resource Utilization (ICRU), both in the emergency and elective setting [8]. Therefore, the potential for postoperative ICU care is an important consideration when selecting patients for cancer surgery during the COVID-19 pandemic.
The purpose of this study was to develop and validate a prediction model and clinical risk score for ICRU after colon cancer surgery, with the goal of facilitating healthcare decisions surrounding medically necessary colon cancer surgery during the COVID-19 pandemic.
Materials and Methods
This prediction model study is reported in accordance with the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) checklist [9].
Patients
Training Set for Model Development
After institutional review board approval, we identified all adult (≥ 18 years old) patients from the 2012 to 2018 ACS-NSQIP colectomy-targeted patient user file (PUF) who underwent an elective colectomy for colon cancer, according to Current Procedural Terminology codes and International Classification of Disease codes. Patient data from the colectomy-targeted PUF was then linked to the general PUF for demographic and general operative and postoperative data, and only patients with data in both databases were further included in the training set. Patients with an American Society of Anesthesiologists (ASA) score ≥ 5 and disseminated cancer or who were preoperatively ventilated or in septic shock were excluded. Patients with missing data on preoperative T-stage, N-stage, operative approach, or ASA score were also excluded.
Test Set for Model Validation
The test set consisted of a similar cohort of patients from the 2012 to 2018 ACS-NSQIP general PUF who underwent an elective colectomy for colon cancer, but who did not have a match in the colectomy-targeted PUF. The same inclusion and exclusion criteria were applied, and the outcome and variables were defined in an identical manner.
Dependent Variable
The primary outcome was 30-day postoperative Intensive Care Resource Utilization (ICRU), defined as a composite outcome including any one of the following postoperative events: (1) unplanned reintubation; (2) ventilator requirements > 48 h; (3) cardiac arrest; (4) septic shock; or (5) death. Unplanned reintubation was defined as the need for endotracheal tube insertion (or similar) and mechanical ventilation for reasons other than re-operation (e.g., refractory hypotension or inability to protect the airway). Septic shock was defined as signs and symptoms of sepsis with documented organ and/or circulatory dysfunction.
Candidate Variables
For the development of a clinically applicable prediction model and risk score, only preoperative factors were considered. The following patient, disease, and treatment factors were considered: age (dichotomized as < 70 and ≥ 70, given the risk for severe SARS-CoV-2 illness in individuals ≥ 70) (6), sex, ASA score (I/II vs. III/IV), obesity (defined as body mass index ≥ 30 kg/m2), diabetes, smoking, cardiorespiratory disease (defined as either chronic obstructive pulmonary disease, congestive heart failure, or dyspnea), hypertension, bleeding disorder, steroid use, preoperative weight loss ≥ 10%, functional status (partially or totally dependent for activities of daily living vs. independent), procedure (right colectomy vs. left colectomy vs. total abdominal colectomy), preoperative chemotherapy within 90 days, tumor depth (early: T1/2 vs. locally advanced: T3/4), nodal status (negative: N0 vs. positive: N1/2), distant metastases, operative approach (minimally invasive surgery (MIS) vs. open surgery), and bowel preparation (any: mechanical and/or oral antibiotic vs. none). Cases of conversion to open surgery were grouped with MIS, as they reflected an operation that the surgeon felt was amenable to an MIS approach. Missing data on bowel preparation was imputed using the Multiple Imputation by Chained Equations (MICE) package in R [10]. No other data was missing, allowing for a complete case analysis.
Model Development and Validation
Patients who required postoperative ICRU were compared with those who did not require ICRU within the training set. Patient, disease, and treatment factors were compared between the two groups on univariate analysis. A multiple logistic regression model was then developed, using a 0.05 forward and backward stepwise selection method for candidate variables. Selected variables in the regression model were then ranked according to their effect size (Wald chi-square statistic) and relative contribution to the overall model, taking into account both the strength of association (odds ratio) and the incidence of the variable. In order to develop a practical clinical risk score with as few variables as possible while retaining the model’s predictive capabilities, variables with an individual contribution of < 5% were dropped; all other variables were retained in the final prediction model. The area under the receiver operating characteristic curve (AUC) (equivalent to the c-statistic) was used to assess for model discrimination and the Hosmer-Lemeshow goodness-of-fit test for model calibration. The final model was then validated in the test set using the same measures of performance as described for model development. A clinical risk score was then developed based on the relative differences in regression coefficients. The risk of postoperative ICRU was displayed across the range of possible points. All data analyses were performed with R v3.5.1 and SAS 9.4.
Sensitivity Analysis
To test the robustness of the clinical risk score, a sensitivity analysis was performed, whereby the following three postoperative events were added to the composite outcome of 30-day postoperative ICRU: pulmonary embolism, pneumonia, and myocardial infarction. While these events do not always require postoperative ICU care, their inclusion may help account for the variability in ICU admission criteria that exists among hospitals. A new model was developed applying the same steps as outlined above, and model discrimination was similarly assessed.
Proof-of-Concept of Clinical Risk Score During COVID-19
To demonstrate the application of the clinical risk score for the selection of elective colon cancer cases during the COVID-19 pandemic, we retrospectively reviewed all planned colon cancer resection at three McGill University–affiliated hospitals from March 13, 2020, to April 20, 2020. Two hospitals (Jewish General Hospital and Montreal General Hospital) were designated level-1/condition-0 hospitals according to the American College of Surgeons Surgery COVID-19 Activation and Response Plan, while one hospital (St. Mary’s Hospital) was in the alert phase [11]. We compared the median clinical risk score among patients who underwent surgery as planned versus those whose who had their surgery plans altered (delayed or referred to a less acute-phase COVID hospital for surgery) due to higher risks of ICRU and mortality from COVID-19, if acquired.
Results
Of 54,893 patients who underwent an elective colon cancer resection in the training set, 655 (1.2%) had an unplanned reintubation, 455 (0.82%) required > 48 h of postoperative ventilation, 221 (0.40%) suffered a cardiac arrest, 524 (0.95%) had septic shock, and 525 (0.96%) died within 30 days of their index surgery. In total, 1224 (2.2%) patients met the definition for postoperative ICRU. In comparison to patients who did not require ICRU, patients who required ICRU were more likely to be ≥ 70 years old (63.3% vs. 40.0%, p < 0.001); had a higher ASA score (III/IV 83.7% vs. 57.9%, p < 0.001); were more likely to have comorbidities such as diabetes (28.7% vs. 18.9%, p < 0.001), cardiorespiratory disease (28.2% vs. 10.5%, p < 0.001), and hypertension (70.5% vs. 52.4%, p < 0.001); and were more likely to be functionally dependent (7.1% vs. 1.5%, p < 0.001). Patients who required ICRU also had more advanced tumors (locally advanced T-stage, 63.7% vs. 58.5%, p < 0.001) and were more likely to undergo open surgery (32.9% vs. 19.6%, p < 0.001) (Table 1).
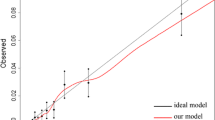
Using stepwise selection, 11 candidate variables were assessed on multiple logistic regression. All variables were significantly associated with the outcome (Table 2). The original model demonstrated good discrimination (AUC = 0.75) and calibration (goodness-of-fit, p = 0.12). Six variables were retained in the final model based on the Wald chi-squared statistic demonstrating an individual contribution to the model of > 5%: age, sex, ASA score, cardiorespiratory disease, functional status, and operative approach (Table 2). Collectively, these 6 variables contributed 91% to the original prediction model, while the 5 excluded variables together contributed 9%. A restricted model was then fit with the 6 retained variables: age (≥ 70; OR 1.90, 95% CI 1.68–2.14), sex (male; OR 1.73, 95% CI 1.54–1.95), ASA score (III/IV; OR 2.52, 95% CI 2.15–2.95), cardiorespiratory disease (yes; OR 2.22, 95% CI 1.94–2.53), functional status (dependent; OR 2.81, 95% CI 2.22–3.56), and operative approach (open surgery; OR 1.70, 95% CI 1.51–1.93) (Table 3). The restricted model maintained good discrimination (AUC = 0.73) and calibration (goodness-of-fit, p = 0.072) (Fig. 1a). Using the regression coefficients, a clinical risk score for postoperative ICRU was developed (Table 3). Based on the possible sum of points, the risk of ICRU ranged from 0.3 (0 points) to 19.0% (8 points) (Table 4).
Given that the final clinical risk score did not include any colectomy-targeted variables, patients from the ACS-NSQIP general PUF who did not have a match within the colectomy-targeted PUF (initially excluded for generation of the training set) could be used for validation. The test set included 33,838 patients who underwent an elective colectomy for colon cancer. With respect to the 6 variables included in the clinical risk score, the training set and test set were reasonably similar (Table 5). A total of 828 (2.5%) patients required 30-day postoperative ICRU in the test set. In applying the training set final prediction model equation to the test set data, the model demonstrated good discrimination (AUC = 0.73) (Fig. 1b).
On sensitivity analysis, the incidence of 30-day postoperative ICRU increased to 4.0% with the inclusion of pulmonary embolism, pneumonia, and myocardial infarction. Model development retained the same 6 variables, and the regression coefficients were similarly distributed to those of the original prediction model to allow for the same point assignment. The sensitivity analysis model maintained good predictive capabilities (AUC = 0.71), and the range of ICRU risk is presented in Table 4.
In total, 27 patients at three McGill University–affiliated hospitals were scheduled for an elective colon cancer resection during the 5-week period affected by the COVID-19 pandemic: 17 patients had their planned operation, while 10 patients had their surgery plans altered due to COVID-19 (7 patients had their operation delayed and 3 patients had their care transferred to a less acute-phase COVID hospital). The median clinical risk score was higher among patients who had their surgery plans altered compared with that among those who had their planned operation (2.5 (1.0–6.0) vs. 1.0 (0.0–2.0), p = 0.004). All three patients with a clinical risk score ≥ 6 (risk of ICRU = 11.9%) had their operations delayed due to COVID-19. None of the operated patients required postoperative ICRU.
Discussion
As the COVID-19 pandemic continues to unfold across North America, the delivery of quality and timely surgical care will remain a challenge. Patients with colon cancer present a particular burden, given the relatively high incidence of the disease, the potential for patients to become acutely symptomatic, and the risk of progression with delays to surgery [4]. Furthermore, no well-established alternative treatment strategy exists for colon cancer, rendering surgery a medically necessary and time-sensitive procedure during the COVID-19 pandemic [5].
In the present study, we have developed and validated a prediction model and clinical risk score for 30-day postoperative ICRU after colon cancer surgery. While other outcomes, such as postoperative complications and length of stay, are important concerns as well, the need for ICRU is one of the most current pressing considerations when contemplating medically necessary cancer surgery. In the American College of Surgeons’ recently published guidelines for the triage of cancer surgery patients, they stated that “for elective cases with a high likelihood of postoperative ICU or respirator utilization, it will be more imperative that the risk of delay to the individual patient is balanced against the imminent availability of these resources for patients with COVID-19” [3]. Using multicenter data from ACS-NSQIP, we reported an overall postoperative ICRU of 2.2% after colon cancer surgery. However, based on a variety of easily obtainable clinical preoperative factors, the risk for ICRU ranged from 0.3 to 19.0%.
Depending on a hospital’s available resources and current phase in the COVID-19 response, this clinical risk score can be used as a tool for surgeons and hospital administrators when deciding on an acceptable postoperative ICRU threshold to proceed with surgery during this dynamic crisis. In reviewing our own institutional data, we observed a higher median clinical risk score among those patients whose surgery plans were altered due to COVID-19. In our hospital system, decisions regarding urgent elective cancer cases are made by a multidisciplinary leadership team on a daily basis [12]. Factors taken into consideration at these daily meetings are available critical resources, urgency of surgery, lack of other suitable treatment alternatives, likelihood to require ICU admission, and risk of acquiring COVID-19 that would result in a poor outcome. Thus, while this small cohort represents only a 5-week snapshot in time and other factors are likely contributory, it highlights the current reality of ICRU importance when making operative decisions during the pandemic and demonstrates the applicability of the clinical risk score.
The 6 variables included in the clinical risk score were mainly patient-level factors, reflecting their overall health status and comorbidities. Many of the included factors are also associated with worse outcomes with SARS-CoV-2 infection, such as older age and comorbidities [7]. Thus, in addition to balancing the need for postoperative ICRU, these factors are also relevant when considering the potential exposure to SARS-CoV-2 associated with hospitalization [13]. Interestingly, none of the cancer-specific variables was included in the final risk score, including the stage of the cancer. This suggests that surgeons may reassuringly prioritize more advanced tumors, so long as the patient’s preoperative functional and medical status is acceptable according to the clinical risk score. Open surgery was also associated with ICRU, and may reflect case complexity. While the majority of colon cancer cases across the USA are performed via an MIS approach [14], open surgery may still be employed in more difficult cases, such as re-operative abdomens. It is less likely that open surgery reflected an intraoperative complication, as cases of unplanned conversion from MIS to open surgery were analyzed according to their initial approach.
There are several strengths to the current study and clinical risk score. The major advantage is the ability to use this clinical risk score at the point of care. The prediction model includes 6 variables, all of which can be easily obtained and/or estimated based on the patient’s medical history and planned operation. The transformation of the prediction model into a simple clinical risk score also allows for easy use when making clinical decisions regarding operative candidacy during COVID-19. The clinical risk score may equally be useful during the recovery phase of the pandemic when operative services resume and a back-log of colon cancer cases needs to be prioritized. The prediction model demonstrated good discrimination and predictive capabilities (AUC = 0.73). The strong performance may be attributed to the robust and data-driven approach to the method of candidate variable selection into the final restricted model. The model was also validated in a separate cohort of similar patients, which is important to assess for any statistical deficiencies during model development and to evaluate the generalizability of the prediction model [15]. Finally, data collection for ACS-NSQIP is prospectively performed by well-trained and audited reviewers who all use detailed prescribed definitions for each variable [16].
There are also several important limitations that must be considered when interpreting the results of this study. First, ICU admission is not a variable in the ACS-NSQIP database, and ICRU was defined as a composite outcome including several pre-defined variables. Therefore, even though the outcomes selected—unplanned reintubation, ventilator requirements > 48 h, cardiac arrest, septic shock, and death—almost always include ICU care at some point in their course, there may be an element of outcome misclassification. To account for this, a sensitivity analysis was performed to include less severe postoperative events that may still require ICU care. The model retained good predictive capabilities on sensitivity analysis. Second, the prediction model was validated in a separate cohort of patients who did not have a match within the colectomy-targeted PUF. There may be some unmeasured differences between the training and test sets, as hospitals that choose to contribute data to the colectomy-targeted PUF may differ from those who do not. Lastly, the clinical risk score is not applicable to rectal cancer patients. In addition to rectal cancer patients having a different risk set, their clinicians can choose from a gamut of therapies including neoadjuvant radiotherapy, chemotherapy, or a combination of these modalities; all of which may downstage the tumor with an acceptable delay to surgery [17]. Patients with colon cancer are typically more in need of immediate surgery. Future work should aim to prospectively validate the clinical risk score in a larger cohort of patients during the COVID-19 era, to better evaluate its application in clinical decision-making during these resource-limited times.
Conclusion
We have developed and validated a prediction model for 30-day postoperative Intensive Care Resource Utilization after colon cancer surgery. The model demonstrated good predictive capabilities and was transformed into a simple clinical risk score to allow for easy use at the point of care. The clinical risk score may aid surgeons and hospital administrators in identifying appropriate colon cancer patients for medically necessary surgery during the COVID-19 pandemic, in an attempt to minimize the use of limited ICU resources.
References
Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TA, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DS, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Moller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493-1505.
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020 Mar 5. doi: https://doi.org/10.3322/caac.21601. Online ahead of print.
American College of Surgeons: COVID-19 guidelines for triage of cancer surgery patients. Available at: https://www.facs.org/covid-19/clinical-guidance/elective-case/cancer-surgery. Accessed April 16, 2020.
Kaltenmeier C, Shen C, Medich DS, Geller DA, Bartlett DL, Tsung A, Tohme S. Time to surgery and colon cancer survival in the United States. Ann Surg. 2019 Dec 10. doi: https://doi.org/10.1097/SLA.0000000000003745. Online ahead of print.
Prachand VN, Milner R, Angelos P, Posner MC, Fung JJ, Agrawal N, Jeevanandam V, Matthews JB. Medically-necessary, time-sensitive procedures: a scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg. 2020 Apr 9. doi: https://doi.org/10.1016/j.jamcollsurg2020.04.011. Online ahead of print.
Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020 Mar 13. doi: https://doi.org/10.1001/jama.2020/4031. Online ahead of print.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062.
Jerath A, Laupacis A, Austin PC, Wunsch H, Wijeysundera DN. Intensive care utilization following major noncardiac surgical procedures in Ontario, Canada: a population-based study. Intensive Care Med. 2018;44:1427-1435.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol. 2015;68:134-143.
Multivariate Imputation by Chained Equations. Package ‘mice’. Available at: https://cran.r-project.org/web/packages/mice/mice.pdf. Accessed April 16, 2020.
Ross SW, Lauer CW, Miles WS, Green JM, Christmas AB, May AK, Matthews BD. Maximizing the calm before the storm: tiered surgical response plan for novel coronavirus (COVID-19). J Am Coll Surg. 2020 Mar 30. doi: https://doi.org/10.1016/j.jamcollsurg.2020.03.019. Online ahead of print.
American College of Surgeons: Create a surgical review committee for COVID-19-related surgical triage decision making. Available at: https://www.facs.org/covid-19/clinical-guidance/review-committee. Accessed April 16, 2020.
Wee LE, Conceicao EP, Sim XYJ, Aung MK, Tan KY, Wong HM, Wijaya L, Tan BH, Ling ML, Venkatachalam I. Minimizing intra-hospital transmission of COVID-19: the role of social distancing. J Hosp Infect. 2020 Apr 12. doi: https://doi.org/10.1016/j.jhin.2020.04.016. Online ahead of print.
Villano AM, Zeymo A, Houlihan BK, Bayasi M, Al-Refaie WB, Chan KS. Minimally invasive surgery for colorectal cancer: hospital type drives utilization and outcomes. J Surg Res. 2020;247:180-189.
Collins GS, de Groot JA, Dutton S, Omar O, Shanyinde M, Tajar A, Voysey M, Wharton R, Yu LM, Moons KG, Altman DG. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol. 2014;14:40.
Shiloach M, Frencher SK Jr, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, Richards KE, Ko CY, Hall BL. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6-16.
Ryan EJ, O’Sullivan DP, Kelly ME, Syed AZ, Neary PC, O’Connell PR, Kavanagh DO, Winter DC, O’Riordan JM. Meta-analysis of the effect of extending the interval after long-course chemoradiotherapy before surgery in locally-advanced rectal cancer. Br J Surg. 2019;106:1298-1310.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work: RG, MAK, ES, DM, AP, NM, SD, ASL, CAV, and MB
Drafting the work or revising it critically for important intellectual content: RG, MAK, ES, DM, AP, NM, SD, ASL, CAV, and MB
Final approval of the version to be published: RG, MAK, ES, DM, AP, NM, SD, ASL, CAV, and MB
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: RG, MAK, ES, DM, AP, NM, SD, ASL, CAV and MB
Corresponding author
Ethics declarations
Conflict of Interest
ASL receives travel stipends from Merck and Servier, and is on the advisory committee of Novadaq. RG, MAK, ES, DM, AP, NM, SD, CAV, and MB have no financial disclosures or conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous presentations/publications: None.
Rights and permissions
About this article
Cite this article
Garfinkle, R., Abou-Khalil, M., Salama, E. et al. Development and Validation of a Clinical Risk Score for Intensive Care Resource Utilization After Colon Cancer Surgery: a Practical Guide to the Selection of Patients During COVID-19. J Gastrointest Surg 25, 252–259 (2021). https://doi.org/10.1007/s11605-020-04665-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-020-04665-9