Abstract
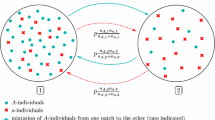
More and more evidence shows that mating preference is a mechanism that may lead to a reproductive isolation event. In this paper, a haploid population living on two patches linked by migration is considered. Individuals are ecologically and demographically neutral on the space and differ only on a trait, a or A, affecting both mating success and migration rate. The special feature of this paper is to assume that the strengths of the mating preference and the migration depend on the trait carried. Indeed, patterns of mating preferences are generally asymmetrical between the subspecies of a population. I prove that mating preference interacting with frequency-dependent migration behavior can lead to a reproductive isolation. Then, I describe the time before reproductive isolation occurs. To reach this result, I use an original method to study the limiting dynamical system, analyzing first the system without migration and adding migration with a perturbation method. Finally, I study how the time before reproductive isolation is influenced by the parameters of migration and of mating preferences, highlighting that large migration rates tend to favor types with weak mating preferences.





Similar content being viewed by others
References
Athreya KB, Ney PE (1972) Branching processes. Springer, Berlin
Berger M, Gostiaux B (1992) Géométrie différentielle: variétés, courbes et surfaces. Presses Universitaires de France
Blaustein AR (1983) Kin recognition mechanisms: phenotypic matching or recognition alleles? Am Nat 121(5):749–754
Bolker B, Pacala SW (1997) Using moment equations to understand stochastically driven spatial pattern formation in ecological systems. Theor Popul Biol 52(3):179–197
Boughman JW (2001) Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411(6840):944–948
Brown JS, Pavlovic NB (1992) Evolution in heterogeneous environments: effects of migration on habitat specialization. Evolut Ecol 6(5):360–382
Bürger R, Schneider KA (2006) Intraspecific competitive divergence and convergence under assortative mating. Am Natur 167(2):190–205
Clobert J, Danchin E, Dhondt AA, Nichols JD (2001) Dispersal. Oxford University Press, Oxford
Collet P, Méléard S, Metz JA (2011) A rigorous model study of the adaptive dynamics of mendelian diploids. J Math Biol 67(3):569–607
Coron C, Costa M, Leman H, Smadi C (2018) A stochastic model for speciation by mating preferences. J Math Biol 76(6):1421–1463
Cuevas J, Moya A, Elena S (2003) Evolution of rna virus in spatially structured heterogeneous environments. J Evolut Biol 16(3):456–466
Dieckmann U, Law R (2000) Relaxation projections and the method of moments. In: Dieckmann U, Law R, Metz JAJ (eds) The geometry of ecological interactions: symplifying spatial complexity. Cambridge University Press, Cambridge, pp 412–455
Elena SF, Agudelo-Romero P, Lalić J (2009) The evolution of viruses in multi-host fitness landscapes. Open Virol J 3:1–6
Ethier SN, Kurtz TG (1986) Markov processes: characterization and convergence. Wiley, New York
Fournier N, Méléard S (2004) A microscopic probabilistic description of a locally regulated population and macroscopic approximations. Ann Appl Probab 14(4):1880–1919
Gavrilets S (2004) Fitness landscapes and the origin of species (MPB-41), vol 41. Princeton University Press, Princeton
Gavrilets S (2014) Models of speciation: Where are we now? J Hered 105(S1):743–755
Gavrilets S, Boake CR (1998) On the evolution of premating isolation after a founder event. Am Natur 152(5):706–716
Gregorius HR (1989) Characterization and analysis of mating systems. Ekopan Verlag, Witzenhausen
Hartl DL, Clark AG, Clark AG (1997) Principles of population genetics, vol 116. Sinauer Associates, Sunderland
Heinzmann D (2009) Extinction times in multitype Markov branching processes. J Appl Probab 46(1):296–307
Hollocher H, Ting CT, Pollack F, Wu CI (1997) Incipient speciation by sexual isolation in drosophila melanogaster: variation in mating preference and correlation between sexes. Evolution 51:1175–1181
Hoppensteadt FC (1966) Singular perturbations on the infinite interval. Trans Am Math Soc 123:521–535
Jones A, Ratterman N (2009) Mate choice and sexual selection: What have we learned since darwin? PNAS 106(1):10,001–10,008
Jugovic J, Crne M, Luznik M (2017) Movement, demography and behaviour of a highly mobile species: a case study of the black-veined white, aporia crataegi (lepidoptera: Pieridae). Eur J Entomol 114:113
Karlin S, McGregor J (1972) Polymorphisms for genetic and ecological systems with weak coupling. Theor Popul Biol 3(2):210–238
Kirkpatrick M (1982) Sexual selection and the evolution of female choice. Evolution 41:1–12
Kondrashov AS, Shpak M (1998) On the origin of species by means of assortative mating. Proc R Soc Lond B Biol Sci 265(1412):2273–2278
Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proc Nat Acad Sci 78(6):3721–3725
Lande R, Kirkpatrick M (1988) Ecological speciation by sexual selection. J Theor Biol 133(1):85–98
LaSalle JP (1960) Some extensions of liapunov’s second method. Trans Circuit Theory IRE 7(4):520–527
Merrill RM, Wallbank RWR, Bull V, Salazar PCA, Mallet J, Stevens M, Jiggins CD (2012) Disruptive ecological selection on a mating cue. Proc R Soc Lond B Biol Sci 279(1749):4907–4913
M’Gonigle LK, Mazzucco R, Otto SP, Dieckmann U (2012) Sexual selection enables long-term coexistence despite ecological equivalence. Nature 484(7395):506–509
O’Donald P (1960) Assortive mating in a population in which two alíeles are segregating. Heredity 15:389–396
Panhuis TM, Butlin R, Zuk M, Tregenza T (2001) Sexual selection and speciation. Trends Ecol Evol 16(7):364–371
Payne R, Krakauer D (1997) Sexual selection, space, and speciation. Evolution 51(1):1–9
Rudnicki R, Zwoleński P (2015) Model of phenotypic evolution in hermaphroditic populations. J Math Biol 70(6):1295–1321
Ruelle D (1989) Elements of differentiable dynamics and bifurcation theory. Academic Press Inc, Boston
Servedio M, Bürger R (2014) The counterintuitive role of sexual selection in species maintenance and speciation. Proc Nat Acad Sci 111(22):8113–8118
Servedio M, Bürger R (2015) The effects of sexual selection on trait divergence in a peripheral population with gene flow. Evolution 69(10):2648–2661
Servedio MR (2011) Limits to the evolution of assortative mating by female choice under restricted gene flow. Proc R Soc Lond B Biol Sci 278(1703):179–187
Smadi C, Leman H, Llaurens V (2018) Looking for the right mate in diploid species: How does genetic dominance affect the spatial differentiation of a sexual trait? J Theor Biol 447:154–170
Smadja C, Ganem G (2005) Asymmetrical reproductive character displacement in the house mouse. J Evolut Biol 18(6):1485–1493
Smadja C, Catalan J, Ganem G (2004) Strong premating divergence in a unimodal hybrid zone between two subspecies of the house mouse. J Evolut Biol 17(1):165–176
Uy JAC, Patricelli GL, Borgia G (2001) Complex mate searching in the satin bowerbird ptilonorhynchus violaceus. Am Nat 158(5):530–542
Van Doorn G, Noest A, Hogeweg P (1998) Sympatric speciation and extinction driven by environment dependent sexual selection. Proc R Soc Lond B Biol Sci 265(1408):1915–1919
Wu CI, Hollocher H, Begun DJ, Aquadro CF, Xu Y, Wu ML (1995) Sexual isolation in drosophila melanogaster: a possible case of incipient speciation. Proc Nat Acad Sci 92(7):2519–2523
Acknowledgements
I would like to thank Pierre Collet for his help on the theory of dynamical systems. I also thank two anonymous reviewers for their constructive comments, which helped me to improve the manuscript. This work was partially funded by the Chair “Modélisation Mathématique et Biodiversité” of VEOLIA-Ecole Polytechnique-MNHN-F.X.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Dynamical System Without Migration
In this appendix, we will prove the results of Sect. 3.2, which is related to the case without migration. To this aim, we use the two following weighted quantities
From (12), we find that
Proof of Lemma 3
We start by studying the stability of equilibrium (0, 0). Assume that \(\varSigma (0)>0\). From (24), we derive
Since \(\varSigma ^2-2(\beta _A-1)(\beta _a-1)z_az_A \ge 0\) and \(-(\beta _{\min }-1)(z_A+z_a) \ge -\varSigma \), we deduce that
Hence, as long as \(\varSigma \in ]0,(b-d)(\beta _{\min }-1)/c[\), \(\varSigma (t)\) is increasing. Thus (0, 0) is an unstable equilibrium.
The stability of the three other equilibria, \((\zeta _A,0)\), \((0,\zeta _a)\) and \((\chi _A,\chi _a)\), can be deduce by a direct computation of Jacobian matrices at these points, which we do not detail.
Finally, let us study the long-time behavior of any solution. Equation (23) implies that the sign of \(\varOmega (t)\) is equal at all time and, that \({\mathcal {D}}^A_0\) is a positively invariant set under dynamical system (12). Moreover, there exists only a stable equilibrium that belongs to the set \({\mathcal {D}}^A_0\), which is \((\zeta _A,0)\).
We consider the function \(W: {\mathcal {D}}^A_0 \rightarrow \mathbb {R}\):
From (23) and (24), we deduce that
Moreover for any \((z_A,z_a)\in {\mathcal {D}}^A_0\), \(W(z_A,z_a)=0\) if and only if \(z_a=0\). \(W(z_A,z_a)\) converges to \(+\,\infty \) when \((\beta _A-1)z_A-(\beta _a-1)z_a\) converges to 0 and \(\frac{dW}{dt}\) is non-positive on \({\mathcal {D}}^A_0\) and is equal to zero if and only if \(z_a=0\). It ensures that W is a Lyapunov function for (12) on the set \({\mathcal {D}}^A_0\) which cancels only on \({\mathcal {D}}^A_0 \cap \{z_a=0\}\). Furthermore, a simple computation gives that the largest invariant set in \({\mathcal {D}}^A_0 \cap \{z_a=0\}\) is \(\{(\zeta _A,0)\}\). Theorem 1 of LaSalle (1960) is thus sufficient to conclude that any solution of (12) with initial condition in \({\mathcal {D}}^A_0\) converges to \((\zeta _A,0)\) when t tends to \(+\,\infty \). Similarly, we prove that any solution with initial condition in \( {\mathcal {D}}^a_0 \) converges to \((0,\zeta _a)\).
Finally, assume that \(\varOmega (0)=0\). Then, \(\varOmega (t)=0\) for all \(t\ge 0\) according to (23) and, in addition with (12), we derive for all \(\alpha \in \mathcal {A}\),
We deduce the last point of Lemma 3 easily. \(\square \)
Appendix B: Extinction Time
This subsection is devoted to the proof of Theorem 2 following ideas similar to the ones of the proof of Theorem 3 and Proposition 4.1 in Coron et al. (2018). Hence, we do not give all details, but explain only parts that are different.
Assume that \(m_A\le m_0\), \(m_a\le m_0\) and that \(\mathbf{{Z}}^K(0)\) converges in probability to a deterministic vector \(\mathbf{{z}^0}\) belonging to \({\mathcal {D}}^{A,a}_{m_A,m_a}\), Lemma 3 and Theorem 1 ensure that \((\mathbf{{Z}}^K(t),t\ge 0)\) reaches a neighborhood of the equilibrium \((\zeta _A,0,0,\zeta _a)\) after a finite time independent from K. Indeed, the process dynamics is close to the one of the limiting deterministic system (4).
To prove Theorem 2, it remains to estimate the time before all a-individuals in patch 1 and all A-individuals in patch 2 disappear. We denote it by
and we assume that the process is initially close to equilibrium \((\zeta _A,0,0,\zeta _a)\). The estimation is deduced from the following Lemma.
Lemma 4
There exist two positive constants \(\varepsilon _0\) and \(C_0\) such that for any \(\varepsilon \le \varepsilon _0\), if there exists \(\eta \in ]0,1/2[\) that satisfies \(\max (|z_{A,1}^0-\zeta _A|,|z_{a,2}^0-\zeta _a|) \le \varepsilon \) and \(\eta \varepsilon /2 \le z_{a,1}^0,z_{A,2}^0 \le \varepsilon /2\), then
Proof
Following the first step of Proposition 4.1’s proof given by Coron et al. (2018), we prove that as long as the population processes \(Z_{a,1}^K(t)\) and \(Z_{A,2}^K(t)\) have small values, the processes \(Z_{A,1}^K(t)\) and \(Z_{a,2}^K(t)\) stay close to \(\zeta _A\) and \(\zeta _a\) respectively.
Then, by bounding death rates, birth rates and migration rates of \((Z^K_{a,1}(t),t\ge 0)\) and \((Z^K_{A,2}(t),t\ge 0)\), we are able to compare the dynamics of these two processes with the ones of
where \(({\mathcal {N}}_a(t),{\mathcal {N}}_A(t)) \in \mathbb {N}^{\{a,A\}}\) is a two types of branching process with types a and A and for which
-
any \(\alpha \)-individual gives birth to a \(\alpha \)-individual at rate b,
-
any \(\alpha \)-individual gives birth to a \(\bar{\alpha }\)-individual at rate \(m_{\bar{\alpha }}\),
-
any \(\alpha \)-individual dies at rate \(b\beta _{\bar{\alpha }}+m_\alpha \).
The goal is thus to estimate the extinction time of such a subcritical two types of branching process. Let M(t) be the mean matrix of the multitype process, that is,
and let G be the infinitesimal generator of the semigroup \(\{M(t),t\ge 0\}\). From the book of Athreya and Ney (1972) p.202, we deduce a formula of G which is
Applying Theorem 3.1 of Heinzmann (2009), we find that
where \(c_a, c_A\) are two positive constants and r is the largest eigenvalue of the matrix G. With a simple computation, we find that \(r=-\omega (A,a)\). From (27), we deduce that the extinction time is of order \(\omega (A,a)^{-1}\log K\) when K tends to \(+\,\infty \) by arguing as in step 2 of Proposition 4.1’s proof of Coron et al. (2018). This concludes the proof of Lemma 4. \(\square \)
Finally, this gives all elements to induce Theorem 2.
Rights and permissions
About this article
Cite this article
Leman, H. A Stochastic Model for Reproductive Isolation Under Asymmetrical Mating Preferences. Bull Math Biol 80, 2502–2525 (2018). https://doi.org/10.1007/s11538-018-0479-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0479-2
Keywords
- Mating preference
- Asymmetrical preference
- Birth-death stochastic model
- Dynamical system
- Long-time behavior
- Perturbation method