Abstract
Purpose
The aim of this study is to assess the variability of 2-deoxy-2-[18F]fluoro-d-glucose ([18F]-FDG) and 3′-deoxy-3′-[18F]-fluorothymidine ([18F]-FLT) uptake in pre-clinical tumor models and examine the relationship between imaging data and related histological biomarkers.
Procedures
[18F]-FDG and [18F]-FLT studies were carried out in nine human tumor xenograft models in mice. A selection of the models underwent histological analysis for endpoints relevant to radiotracer uptake. Comparisons were made between in vitro uptake, in vivo imaging, and ex vivo histopathology data using quantitative and semi-quantitative analysis.
Results
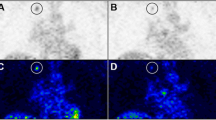
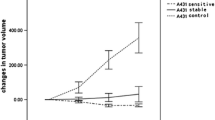
In vitro data revealed that [1-14C]-2-deoxy-d-glucose ([14C]-2DG) uptake in the tumor cell lines was variable. In vivo, [18F]-FDG and [18F]-FLT uptake was highly variable across tumor types and uptake of one tracer was not predictive for the other. [14C]-2DG uptake in vitro did not predict for [18F]-FDG uptake in vivo. [18F]-FDG SUV was inversely proportional to Ki67 and necrosis levels and positively correlated with HKI. [18F]-FLT uptake positively correlated with Ki67 and TK1.
Conclusion
When evaluating imaging biomarkers in response to therapy, the choice of tumor model should take into account in vivo baseline radiotracer uptake, which can vary significantly between models.






Similar content being viewed by others
References
Hawkins RA, Choi Y, Huang SC, Messa C, Hoh CK, Phelps ME (1992) Quantitating tumor glucose metabolism with FDG and PET. J Nucl Med 33(3):339–344
Warburg O (1931) The metabolism of tumours. Smith, New York, pp 129–169
Som P, Atkins HL, Bandoypadhyay D et al (1980) A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med 21(7):670–675
Boellaard R, O'Doherty MJ, Weber WA et al (2010) FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 37(1):181–200
Hoekstra OS, Ossenkoppele GJ, Golding R et al (1993) Early treatment response in malignant lymphoma, as determined by planar fluorine-18-fluorodeoxyglucose scintigraphy. J Nucl Med 34(10):1706–1710
Eary JF, O’Sullivan F, Powitan Y, Chandhury KR, Bruckner JD, Conrad EU (2002) Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging 29(9):1149–1154
Weber WA (2010) Monitoring tumor response to therapy with 18F-FLT PET. J Nucl Med 51(6):841–844
Benz MR, Czernin J, Tap WD et al (2010) FDG-PET/CT imaging predicts histopathologic treatment responses after neoadjuvant therapy in adult primary bone sarcomas. Sarcoma 2010:143540, Epub 2010 Apr 18
Weber WA (2009) Assessing tumor response to therapy. J Nucl Med 50(Suppl 1):1S–10S
Bading JR, Shields AF (2008) Imaging of cell proliferation: status and prospects. J Nucl Med 49(Suppl 2):64S–80S
Shah C, Miller TW, Wyatt SK et al (2009) Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin Cancer Res 15(14):4712–4721
Sohn HJ, Yang YJ, Ryu JS et al (2008) [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res 14(22):7423–7429
Leyton J, Alao JP, Da Costa M et al (2006) In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3’-deoxy-3’-[18F]fluorothymidine positron emission tomography. Cancer Res 66((15) (18)):9178–9185
Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO (2007) Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3’-deoxy-3’-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging 34:1339–1347
Waldherr C, Mellinghoff IK, Tran C et al (2005) Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3’-deoxy-3’-18F-fluorothymidine PET. J Nucl Med 46:114–120
Pio BS, Park CK, Pietras R et al (2006) Usefulness of 3’-[F-18]fluoro-3’-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol 8:36–42
Troost EG, Bussink J, Hoffmann AL, Boerman OC, Oyen WJ, Kaanders JH (2010) 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med 51(6):866–874
Solit D, Santos E, Pratilas DA, et al (2007) 3-Deoxy-3-[18F]fluorothymidine positron emission tomography is a sensitive method for imaging the response of BRAF dependent tumors to MEK inhibition. Cancer Res 67(23):11463–11469
Brepoels L, Stroobants S, Verhoef G, De Groot T, Mortelmans L, De Wolf-Peeters C (2009) (18)F-FDG and (18)F-FLT uptake early after cyclophosphamide and mTOR inhibition in an experimental lymphoma model. J Nucl Med 50(7):1102–1109
Shinto A, Nair N, Dutt A, Baghel NS (2008) Early response assessment in gastrointestinal stromal tumors with FDG PET scan 24 hours after a single dose of imatinib. Clin Nucl Med 33(7):486–487
Gu J, Yamamoto H, Fukunaga H, Danno K et al (2006) Correlation of GLUT-1 overexpression, tumor size, and depth of invasion with 18F-2-fluoro-2-deoxy-D-glucose uptake by positron emission tomography in colorectal cancer. Dig Dis Sci 51(12):2198–2205
Juweid ME, Cheson BD (2006) Positron-emission tomography and assessment of cancer therapy. N Engl J Med 354(5):496–507
Wahl RL, Hutchins GD, Buchsbaum DJ et al (1991) 18F-2-deoxy-2-fluoro-D-glucose uptake into human tumor xenografts. Feasibility studies for cancer imaging with positron-emission tomography. Cancer 67(6):1544–1550
Ebenhan T, Honer M, Ametamey SM et al (2009) Comparison of [18F]-tracers in various experimental tumor models by PET imaging and identification of an early response biomarker for the novel microtubule stabilizer patupilone. Mol Imaging Biol 11(5):308–321
Hendzel MJ, Wei Y, Mancini MA et al (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106(6):348–360
Ladstein G, Bachmann IM, Straume O, Akslen LA (2010) Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer 10:140
Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182(3):311–322
Chung JK, Lee YJ, Kim SK, Jeong JM, Lee DS, Lee MC (2004) Comparison of [18F]fluorodeoxyglucose uptake with glucose transporter-1 expression and proliferation rate in human glioma and non-small-cell lung cancer. Nucl Med Commun 25(1):11–17
Hamacher K, Coenen HH, Stöcklin G (1986) Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 27:235–238
Reischl G, Blocher A, Wei R et al (2006) Simplified, automated synthesis of 3'-[18F]fluoro-3'-deoxy-thymidine ([18F]FLT) and simple method for metabolite analysis in plasma. Radiochim Acta 94:447–451
Kim JS, Lee JS, Im KC, et al (2007) Performance measurement of the microPET focus 120 scanner. J Nucl Med 48(9):1527–1535
Bao Q, Newport D, Chen M, Stout DB, Chatziioannou AF (2009) Performance evaluation of the Inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. J Nucl Med 50:401–408
Gambhir S (2004) Quantitative assay development for pet (chapter 2). In: Phelps ME (ed) PET: molecular imaging and its biological applications. Springer, Berlin
McKay JS, Bigley A, Bell A et al (2006) A pilot evaluation of the use of tissue microarrays for quantitation of target distribution in drug discovery pathology. Exp Toxicol Pathol 57(3):181–193
Smith NR, James NH, Oakley I et al (2007) Acute pharmacodynamic and antivascular effects of the vascular endothelial growth factor signaling inhibitor AZD2171 in Calu-6 human lung tumor xenografts. Mol Cancer Ther 6(8):2198–2208
Hickinson DM, Klinowska T, Speake G et al (2010) AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: a unique agent for simultaneous ERBB receptor blockade in cancer. Clin Cancer Res 16(4):1159–1169
Kim SL, Kim EM, Cheong SJ et al (2009) The effect of PPAR-gamma agonist on (18)F-FDG uptake in tumor and macrophages and tumor cells. Nucl Med Biol 36(4):427–433
Lutz AM, Ray P, Willmann JK, Drescher C, Gambhir SS (2007) 2-Deoxy-2-[F-18]fluoro-D-glucose accumulation in ovarian carcinoma cell lines. Mol Imaging Biol 9(5):260–266
Wang H, Zhang J, Tian J et al (2009) Using dual-tracer PET to predict the biologic behavior of human colorectal cancer. J Nucl Med 50(11):1857–1864
Waki A, Katoa H, Yanoa R et al (1998) The importance of glucose transport activity as the rate-limiting step of 2-deoxyglucose uptake in tumor cells in vitro. Nucl Med Biol 25(7):593–597
Kallinowski F, Schlenger KH, Runkel S et al (1989) Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res 49(14):3759–3764
Bergstrom M, Monazzam A, Razifar P, Ide S, Josephsson R, Langstrom B (2008) Modeling spheroid growth, PET tracer uptake, and treatment effects of the Hsp90 inhibitor NVP-AUY922. J Nucl Med 49(7):1204–1210
Jin Q, Agrawal L, Vanhorn-Ali Z, Alkhatib G (2006) GLUT-1-independent infection of the glioblastoma/astroglioma U87 cells by the human T cell leukemia virus type 1. Virology 353(1):99–110
Haberkorn U, Ziegler SI, Oberdorfer F et al (1994) FDG uptake, tumor proliferation and expression of glycolysis associated genes in animal tumor models. Nucl Med Biol 21(6):827–834
Hamada K, Tomita Y, Qiu Y et al (2008) 18F-FDG-PET of musculoskeletal tumors: a correlation with the expression of glucose transporter 1 and hexokinase II. Ann Nucl Med 22(8):699–705
de Geus-Oei LF, van Krieken JH, Aliredjo RP et al (2007) Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer 55(1):79–87
Seo S, Hatano E, Higashi T et al (2009) P-glycoprotein expression affects 18F-fluorodeoxyglucose accumulation in hepatocellular carcinoma in vivo and in vitro. Int J Oncol 34(5):1303–1312
van Waarde A, Been LB, Ishiwata K, Dierckx RA, Elsinga PH (2006) Early response of sigma-receptor ligands and metabolic PET tracers to 3 forms of chemotherapy: an in vitro study in glioma cells. J Nucl Med 47(9):1538–1545
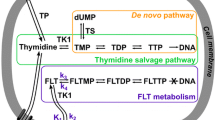
McKinley E, Guleryuz S, Zhao P et al (2010) Thymidine salvage to reflect tumor cell proliferation: Implications for 18F-FLT PET as a biomarker in oncology. J Nucl Med 51:446, MEETING ABSTRACTS
Buck AK, Bommer M, Stilgenbauer S et al (2006) Molecular imaging of proliferation in malignant lymphoma. Cancer Res 66(22):11055–11061
Buck AK, Kratochwil C, Glatting G et al (2007) Early assessment of therapy response in malignant lymphoma with the thymidine analogue [18F]FLT. Eur J Nucl Med Mol Imaging 34(11):1775–1782
Schwartz JL, Tamura Y, Jordan R, Grierson JR, Krohn KA (2003) Monitoring tumor cell proliferation by targeting DNA synthetic processes with thymidine and thymidine analogs. J Nucl Med 44(12):2027–2032
Workman P, Twentyman P, Balkwill F et al (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) Guidelines for the Welfare of Animals in Experimental Neoplasia (Second Edition). Br J Cancer 77(1):1–10
Acknowledgements
We thank the Radiopharmacy of the University Hospital of Tübingen for providing the radiotracers. We also thank John Foster, Alison Bigley, and Neil Gray of AstraZeneca for providing immunohistochemistry support.
Conflict of Interest
All work performed was funded by AstraZeneca. However, since no AstraZeneca product was evaluated in this study, this does not create any conflict of interest. Bernd Pichler has consulted for AstraZeneca, BayerHealthcare, Boehringer-Ingelheim, and Siemens Healthcare in the last 3 years. All other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ethics Statement
Animal experiments were performed according to the European ethical guidelines of animal experimentation, and in compliance with and the United Kingdom Co-ordinating Committee on Cancer Research guidelines [53]. The animal care unit at Oncodesign is authorized by the French ministries of Agriculture and Research (Agreement No. A21231011). All procedures with animals carried out at Oncodesign and the University of Tübingen were approved by the Animal Care and Use Committee of Pharmacy and Medicine University (Dijon) and were submitted to the ethical committee of the University of Tübingen (Tübingen, Germany). Alderley Park studies were carried out in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986.
Rights and permissions
About this article
Cite this article
Keen, H., Pichler, B., Kukuk, D. et al. An Evaluation of 2-deoxy-2-[18F]Fluoro-D-Glucose and 3′-deoxy-3′-[18F]-Fluorothymidine Uptake in Human Tumor Xenograft Models. Mol Imaging Biol 14, 355–365 (2012). https://doi.org/10.1007/s11307-011-0504-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0504-4