Abstract
It has been estimated that more than 48% of global methane emissions from lakes and reservoirs occur at low latitudes (<24°). To improve this estimate, knowledge regarding underexplored ecosystems, particularly deep lakes and reservoirs in Asian monsoon regions, is needed because the magnitude of methane emissions is influenced by lake bathymetry and climatic conditions. We conducted long-term studies beginning in 2004 at Feitsui Reservoir (FTR) in Taiwan, a subtropical monomictic system with a maximum depth of 120 m to monitor seasonal and interannual variations of three key characteristics and to understand the mechanisms underlying these variations. Key characteristics investigated were as follows: (1) the balance of primary production and heterotrophic respiration as a determinant of vertical oxygen distribution, (2) methane production at the bottom of the reservoir, oxidation in the water column, and emissions from the lake surface, and (3) the contribution of methane-originated carbon to the pelagic food web through methane-oxidizing bacteria (MOB). This review highlights major achievements from FTR studies integrating isotopic, microbial, and modeling approaches. Based on our findings, we proposed two conceptual models: (1) a model of methane dynamics, which addresses the differences in methane emission mechanisms between deep and shallow lakes, and (2) a spatially explicit model linking benthic methane production to the pelagic food web, which addresses the diversity of MOB metabolisms and their dependence on oxygen availability. Finally, we address why long-term studies of subtropical lakes and reservoirs are important for better understanding the effects of climate on low- to mid-latitude ecosystems.
Similar content being viewed by others
Background
Growing evidence indicates that the global methane budget may be influenced by methane release from freshwater systems (e.g., Bastviken et al. 2004; Ciais et al. 2013; Hamdan and Wickland 2016) and shallow coastal areas in marine systems (Borges et al. 2016, 2017), whereas the open ocean (excluding areas with hydrates, especially in the arctic) is a minor contributor (Bates et al. 1996; Rhee et al. 2009). Freshwater studies encompassing arctic (Kling et al. 1992; Laurion et al. 2010), boreal (Bastviken et al. 2004; Huttunen et al. 2003), and temperate (e.g., Michmerhuizen et al. 1996) systems, have led to an estimated emission of 103 Tg methane year−1 from lakes, reservoirs, and rivers (Bastviken et al. 2011). This estimate, which is equal to 0.65 Pg of C (expressed as CO2 equivalent) and 25% of the estimated terrestrial greenhouse gas sink, would be even larger if more recent studies of rivers were considered (Borges et al. 2015a, b).
The magnitude of methane emissions from lake water surfaces is largely influenced by climate and lake bathymetry (i.e., depth and area) (Bastviken et al. 2004), which critically determine the vertical distribution of oxygen. For example, the duration of the thermal stratification period and lake bathymetry control the depth and stratification intensity of the mixed layer (Wilhelm and Adrian 2008), which, in turn, determine the balance of primary production (PP) and aerobic respiration (Ostrom et al. 2005), especially in surface layers. This balance affects the degree of oxygen depletion in deeper layers, which controls the production and oxidation of methane because these processes are regulated by oxygen availability (Murase et al. 2005). For these reasons, regional variations in freshwater methane emissions are important factors that must be considered to ensure reliable global estimates (Bastviken et al. 2004; Tranvik et al. 2009; Pacheco et al. 2013).
According to estimates based on several studies, more than 48% of global methane emissions from lakes and reservoirs are due to methane released at lower latitudes (<24°) (calculated from Table 1 in Bastviken et al. 2011). However, most studies of methane emissions from lakes and reservoirs at lower latitudes concerned shallow lakes (i.e., Amazon floodplains; Bastviken et al. 2010), only five of which were located in Asia. Although this distribution is partly reasonable because most lakes are shallow (Wetzel 1990), more studies of deep lakes and reservoirs in Asian monsoon regions will improve the accuracy of such estimates.
Multiple factors influence methane biogeochemistry, with key determinants of methane oxidation being oxygen availability, oxygen-to-substrate ratio (Morana et al. 2015), and temperature (Lofton et al. 2014). The main determinants of anaerobic methane production are oxygen deficiency and substrate availability, which are also influenced by lake bathymetry (Bastviken et al. 2004, 2008). Anaerobic methane oxidation is believed to be coupled with denitrification, which is affected by nitrogen availability (Deutzmann et al. 2014). The phylogeny of archaeal and bacterial groups indicates their specific roles in methane production and oxidation (Borrel et al. 2011).
In addition to their role in biogeochemical cycling, methane-oxidizing bacteria (MOB), also known as methanotrophs, represent alternative carbon resources at higher trophic levels in benthic and pelagic food webs (Kiyashko et al. 2001; Deines and Fink 2011; Jones and Grey 2011). The contribution of methane-derived carbon via MOB to the pelagic food web changes seasonally in temperate regions (Taipale et al. 2009). However, this topic remains underexplored for lakes and reservoirs in subtropical and tropical regions (hereinafter referred to as “lower-latitude regions”).
Due to high water temperature and meromixis, tropical lakes have high potential for methane production in anoxic deep waters and sediment (e.g., Abril et al. 2005; Pasche et al. 2011), resulting in characteristic methane accumulation near the bottom under reducing conditions. In contrast, monomictic subtropical lakes can recover from hypoxia in deep water by vertical mixing in winter or extreme weather events such as typhoons (e.g., Tanaka and Tsuda 1996; Yoshimizu et al. 2010) and hurricanes. This effect may decrease the potential for methane production by methanogens in the sediment, but can facilitate methane oxidation by MOB in the water column. Therefore, an understanding of these processes in subtropical deep lakes will provide insight into the mechanisms underlying the carbon budget, methane emissions, and MOB roles in food webs of lakes and reservoirs at lower latitudes, and will help improve estimates of global carbon and methane release.
This study was conducted to provide information to enable a better understanding of the mechanisms of methane dynamics at lower latitudes. First, we highlight the research questions, approaches, and some achievements from a long-term study of a subtropical deep reservoir. Second, we propose some perspectives, including a revised conceptual model for methane dynamics in lakes and reservoirs at lower latitudes and new research directions.
Research questions and approaches
With the aim of improving understanding of methane dynamics at lower latitudes, we specifically focused on three questions. (1) How do physical structure and seasonal disturbances alter the balance between PP and bacterial heterotrophy? (2) Under what conditions would methane production and oxidation be enhanced in lower-latitude lakes? (3) Under what conditions would the contribution of MOB to the food web increase? An answer to the first question would help address the subsequent two questions because these two counter biological processes (PP and bacterial heterotrophy) control the redox conditions that subsequently affect methane dynamics.
To address our research questions, we conducted a multiyear survey in which we observed the PP, aerobic respiration, and dynamics of methane in response to environmental changes. This study was motivated by the notion that small lake systems are particularly sensitive to different environmental conditions with interannual variations in climate.
Beginning in November 2004, we conducted field sampling at Feitsui Reservoir (FTR) (120.34E, 24.54N; maximal depth 120 m) in northern Taiwan. The FTR is a good model system for deep monomictic lakes in subtropical regions because (1) it is well-protected from anthropogenic pollution and therefore habitat destruction, and its nutrient status is oligotrophic to mesotrophic (Chang and Wen 1997); and (2) the region has substantial interannual variations in winter mixing intensity, degree of summer stratification, and thickness of hypoxic hypolimnion depending on weather conditions (Itoh et al. 2015; Ho et al. 2016). In addition, typhoons are a major disturbance of summer stratification in this region (e.g., Fan and Kao 2008). Extreme weather events such as typhoons, which typically occur at lower latitudes, affect water and material cycling in lakes. The strength and frequency of typhoons passing over or near the FTR change interannually; therefore, we can focus on and observe the ecosystem responses to changes in hydrodynamics.
Results of research on the FTR
R1: Disproportionate enhancement of bacterial over algal activity induced by typhoons
Using time-series data from 2004 to 2007, Tseng et al. (2010) showed that the ratio of bacterial production (BP) to PP (hereafter; BP/PP) in the FTR was higher in strong typhoon years (2004 and 2005: 27 ± 40%) than in normal typhoon years (2006 and 2007: 12 ± 9%), indicating a disproportionate increase of BP relative to PP after typhoons. In the FTR, BP was twofold greater, but PP was only 20% greater in strong than in normal/weak typhoon years. Such disproportionate enhancement of heterotrophic bacterial activity by typhoons has seldom been described in freshwater ecosystems. Previous studies only focused on the effects of typhoons on autotrophic activity (PP) (Ko et al. 2016, 2017; and citations therein), whereas only a few studies quantified both PP and BP (Shiah et al. 2000; Tsuchiya et al. 2015).
The increased BP/PP with typhoons in the FTR can be explained by the relative extent of the phosphorus (P) limitation in PP vs. BP. Whereas bioassay experiments demonstrated that autotrophic and heterotrophic activities are limited by P, but not carbon or nitrogen (Tseng et al. 2010), heterotrophic bacteria exhibited a faster response than algae to phosphate enrichment (Fig. 7 in Tseng et al. 2010). These findings suggest that, in the field, the P pulse introduced by typhoons might relieve bacteria from P limitation more than phytoplankton, which fits well with past empirical studies and theories indicating that bacteria are responsible for the major uptake of P due to their superior competition capacity in oligotrophic ecosystems (Currie and Kalff 1984; Thingstad et al. 1997; Vadstein 2000).
Strong typhoons may affect plankton activities in both euphotic and aphotic zones. Heavy rains caused by typhoons resulted in supply of P via hyperpycnal flow. This P entered aphotic zones and enhanced bacterial production in aphotic and euphotic zones. Decoupling of BP and PP (i.e., increased BP/PP) in euphotic zones and increased BP in aphotic zones resulted in enhanced consumption and reduced concentration of dissolved organic carbon (DOC) in euphotic and deeper zones (Tseng et al. 2010).
R2: Distinct interannual variations of dissolved oxygen (DO) vertical profile between summer and winter
The vertical profile of DO is the key factor controlling aerobic and anaerobic respiration. Itoh et al. (2015) found that the interannual DO patterns in summer were different from those in winter in FTR, implying the presence of season-specific controlling mechanisms. In a typical monomictic lake, DO levels in the deep layer should be highest after vertical mixing of the water column in the coolest part of the year and lowest at the end of the stratification period. Dissolved oxygen levels at the bottom during the coolest period were negatively correlated with surface water temperature in 2005–2014 (Itoh et al. 2015). Higher surface water temperature led to weaker winter mixing and deficiency of DO at the bottom, which could last until the next stratification period.
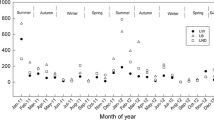
During summer (June–September), when rainfall peaks with summer monsoon fronts and typhoons, interannual variation of DO levels (evaluated by the saturation level required to normalize temperature dependence) was complex and tended to depend on depth. This result was attributed to an increase in lateral water flow from upstream rivers and hillslopes with intensive rainfall and its effects on microbial activities. In the mid-depth layers (20–30 m), DO levels tended to be lower in strong typhoon years (2004 or 2005) than in weak typhoon years (2006 or 2007) (Fig. 1). There were no differences in DO levels at 0, 10, and 50 m among years during summer. In the summer of 2004, typhoons might have induced lateral turbid flow from upstream, with movement of eroded soils or suspended sediments into mid-depth layers (20–50 m) (Fan and Kao 2008). Particles suspended in turbid lateral flow would contribute to oxygen consumption in mid-depth layers. Therefore, these results imply that DO levels at mid-depth layers might be affected by typhoons and subsequent disproportionate increases in bacterial activity over algal growth. However, at the same time, the vertical profile of DO in strong typhoon years (2004 and 2005) indicated that the turbid lateral flow provided external DO to deeper layers (90 m; Fig. 1). Dissolved oxygen levels at the bottom would not only be caused by typhoons, but would also be influenced by the legacy of winter mixing in the previous year (Itoh et al. 2015).
Dissolved oxygen (DO) saturation (%) at each sampling depth from June to September of 2004–2007 (strong typhoon years 2004 or 2005 and weak typhoon years 2006 or 2007). Numbers in parentheses indicate numbers of samples. Box plots show the median (line), 25th–75th percentiles (box), 10th–90th percentiles (bars), and individual values <10th or >90th percentile (points). Differences were detected by one-way ANOVA and Tukey’s multiple comparison tests. Different letters indicate significant differences (P < 0.05)
R3. Role of winter mixing in determining anaerobic methane production in lake sediment
Observations in FTR indicated that: (1) stratification can be maintained, even during winter, when mixing is weak because of high surface water temperature, and (2) lower surface water temperature in winter leads to more intense mixing. Reducing conditions in the bottom layer caused by incomplete vertical mixing in winter decreased profundal DO and NO3 − concentrations the following summer. Oxygen was depleted during the subsequent thermal stratification period, which facilitated NO3 − consumption by denitrifiers. Based on seasonal variations in the vertical profiles of methane concentrations and stable isotope signal (δ13C) values from 2012 to 2014, weak winter mixing can increase sedimentary methane production and therefore profundal methane storage through hypoxia during the thermal stratification period (Itoh et al. 2015). Unfortunately, we did not observe methane dynamics in 2004–2007 and were therefore unable to directly investigate the effects of typhoons on methane production (see section above). This situation is analogous to a study of an Amazonian tropical lake conducted by Marotta et al. (2014), in which winter mixing was not expected. Overall, the results demonstrated that anaerobic biological methane production in the sediments increased exponentially in response to increased temperature.
R4: Effects of methane oxidation in the water column on methane release from the surface
Although a long strong stratification period increased the amount of methane that was produced and accumulated in the bottom layer, this process did not directly enhance the amount of methane emitted from the surface. With a maximum depth of 120 m, the FTR is deep enough so that most methane produced in the profundal layer is consumed and oxidized by MOB (Itoh et al. 2015). Such decoupling of methane accumulation in the bottom layer from methane emission at the surface has also been observed in strongly stratified tropical meromictic lakes (e.g., Borges et al. 2011). Methane oxidation in the water column was evidenced by a decrease in methane concentration with increasing distance from sediment (mostly within 20–30 m above the sediment) in tropical (Rudd 1980; Guérin and Abril 2007; Borges et al. 2011), subtropical, and temperate lakes (Eckert and Conrad 2007; Bastviken et al. 2008; Chanudet et al. 2011; Roland et al. 2017). Other studies directly demonstrated methane oxidation in the water column using water incubation experiments (e.g., Utsumi et al. 1998).
In the FTR, substantial methane oxidation in the water column was initially revealed by investigating the δ13C-methane profile in water samples with low methane concentrations (Itoh et al. 2015). The same method was used in tropical Lake Kivu (Morana et al. 2015). The δ13C-methane values were consistently negative in the near-bottom layer, reflecting that large isotope fractionation occurred during methanogenesis. The 13C-methane values were higher in the oxic/anoxic boundary layer (especially up to 30 m above the sediment surface). These results suggest that much of the enrichment of dissolved 13CH4 was a result of methane oxidation because MOB consume 12CH4 slightly faster than 13CH4. Anaerobic and aerobic methane oxidation would be involved in methane consumption. Even during the stratified period, MOB were the predominant component of the whole bacterial community near the bottom of the water column, where oxygen was almost depleted, as shown by Kojima et al. (2014) using catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH) analysis.
R5: Major types of methanotrophs in FTR
Molecular analysis of bacterial communities in FTR revealed eight species-level operational taxonomic units (OTUs) of Type I MOB (gammaproteobacteria, commonly found in temperate lakes), one OTU of Type II MOB (alphaproteobacterial, commonly found in tropical lakes), and one Methylomirabilis-like OTU belonging to candidate phylum NC10 (Kojima et al. 2014). Methylomirabilis oxyfera is a nitrite-dependent methane oxidizer (Ettwig et al. 2010).
Vertical analysis of 16S rRNA gene-based clone libraries demonstrated that Type I and II MOB were distributed in the hypoxic layer at 90 m, even during the summer stratification period, and in the oxic surface layer at 10 m (Kojima et al. 2014). Clone libraries of pmoA genes encoding particulate methane monooxygenase confirmed their presence at 90 m in the winter. This result is inconsistent with the conventional hypothesis that Type I and II MOB are aerobic. The number of 16S rRNA gene clone libraries analyzed was not sufficient to permit discussion of the seasonal or interannual variations in relative abundances of Type I and II MOB. However, CARD-FISH analysis of bacteria in the 90 m layer during winter (December 2013) demonstrated the dominance of Methylomirabilis-like OTUs (Kojima et al. 2014). These records represent the first evidence of anaerobic methane oxidizers in the water column of lake ecosystems, although many studies have reported the presence of anaerobic methane oxidizers coupled with denitrification in freshwater sediment (Raghoebarsing et al. 2006; Ettwig et al. 2009, 2010; Deutzmann et al. 2014; Norði and Thamdrup 2014).
These results have two implications. First, spatial distributions of Types I and II MOB imply that they are involved in carbon flow under both oxic and hypoxic conditions, relying on distinct biochemical pathways (Vecherskaya et al. 2009; Kits et al. 2015). Second, the presence of the NC10 OTU close to the anaerobic nitrite reducer Cadidatus M. oxyfera implies that methane oxidation would be coupled with nitrogen cycling in the water column of FTR. Further study of anaerobic methane oxidizers in lower-latitude lakes will be needed for a thorough understanding of MOB activities in lake ecosystems.
R6: Interannual variations in the MOB contribution to the pelagic food web
Results of isotope analyses based on the MixSIR Beyesian mixing model demonstrated interesting seasonal and interannual (2010–2013) variations in the contributions of MOB to the pelagic food web (Ho et al. 2016). The MOB contribution tended to be highest in winter, consistent with patterns in other climatic regions, including boreal (Taipale et al. 2011) and tropical lakes (Morana et al. 2015). Interannual MOB contribution variations during winter may be influenced by two contrasting mechanisms. On one hand, deficient profundal DO in summer enhances methanogenesis and accumulation of profundal methane toward winter, supplying more substrate to MOB and resulting in a higher contribution of MOB during winter. However, oxygen supply for profundal waters caused by winter mixing enhances aerobic methane oxidation, resulting in a higher contribution of MOB to the food web during winter. The former mechanism would be the case if MOB were more limited by methane availability than oxygen availability, which is clearly the case in the FTR.
The results of a vertically structured food web model using reaction–advection–diffusion equations predicted that deeper disturbance during summer would suppress the contribution of MOB during winter (Ho et al. 2016). Although winter mixing could have positive and negative effects on the contribution of MOB over the year, stronger mixing in winter resulted in weaker deficiency of profundal DO in the next summer, leading to lower MOB contribution in the following winter. These findings are consistent with the methane accumulation pattern (Itoh et al. 2015) and estimates from the stable-isotope mixing model (Ho et al. 2016).
Molecular analyses of bacterial communities indicated that the taxonomic composition of MOB (i.e., anaerobic MOB phylogenetically close to NC10 and aerobic Types I and II) changed seasonally and vertically (Kojima et al. 2014; Kobayashi et al. 2016). However, because of the limited availability of quantitative data, MOB functional activity in the food web model was parameterized following an earlier experimental study (Harrits and Hanson 1980). The activity of MOB was assumed to be suppressed by low or high oxygen availability and to be highest at an oxygen level of around 200 mmol O2 m−3. This model is a black box approach to represent the diverse functionality of MOB implicitly by assuming that the community is a mixture of aerobic and anaerobic MOB. If we assumed much lower optimal DO levels (with predominance of anaerobic MOB), then the model would be unable to explain the higher contribution of MOB observed in winter than summer when oxygen availability is high because of vertical water mixing. Therefore, we argue that both aerobic and anaerobic reactions are responsible for sustaining food web productivity, especially during winter.
Perspectives
Perspective 1: Vertical distribution of methane in deep lake/reservoir at lower latitudes
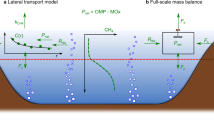
Here, we propose a conceptual model for the vertical patterns of methane and its related elements in a deep lake during the stratification period (Fig. 2). This model includes stable isotope signatures for methane compared with the shallow lake illustration modified from Bastviken et al. (2004).
Schematic of methane dynamics in shallow lakes and coastal parts of deep lakes (modified from Bastviken et al. 2004) and pelagic parts of deep lakes (modified from Itoh et al. 2015) during the stratification period. Right panel shows vertical profiles of temperature, DO, methane concentration, methane carbon isotope ratio, and nitrate concentration at the end of the stratification period (Dec 2013) in FTR (Itoh et al. 2015)
Methane production, originating from organic matter and CO2, mainly occurs in the anaerobic sediment. Production of sedimentary methane (by methanogenesis) and CO2 (by heterotrophic respiration) is controlled by profundal DO. The vertical distribution of DO depends on differences in the intensities of stratification and mixing, which are affected by climatic conditions. Despite the high profundal methane concentration in the stratified period, most of the dissolved methane can be oxidized within 20–30 m above the sediment layer (see “R4: Effects of methane oxidation in the water column on methane release from the surface”) in deep lakes. This fact suggests that sedimentary methane production is not a main source of methane emissions from lakes with sufficient depth (right diagram in Fig. 2). In contrast, methane produced in the sediment of shallow lakes affects methane flux at the water surface as both ebullition and diffusion flux while being oxidized incompletely (left diagram in Fig. 2). This phenomenon is also true for methane produced in sediment of the shallow part of a deep lake. For example, findings in German lakes showed that the ratio of the surface area of the shallow water zone to the entire lake area was a better predictor of surface methane concentration than the total surface area (Encinas Fernández et al. 2016). Nevertheless, studies of the distinct methane dynamics in shallow vs. deep parts of lakes at lower latitudes are needed to confirm the robustness of their conclusion.
Methane emissions from deep lakes could potentially be explained by subsurface methane production. The maximum amount of subsurface methane reported in some oceans and lakes implies in situ methane production in oxic waters (Bogard et al. 2014; Tang et al. 2014; Itoh et al. 2015; Yao et al. 2016). Therefore, subsurface rather than profundal methane production may account for a portion of the methane emitted from the water surface. As frequently occurs in well-stratified tropical lakes (Verburg et al. 2003), cyanobacterial blooms occur during summer in the FTR. It may be that the subsurface cyanobacteria bloom in summer plays a neglected role in the production of methane and the vertical distribution of oxygen and therefore regulates anaerobic methanogenesis in the bottom layer. Although interactions between cyanobacteria and bacteria/archaea can result in methane production in oxic layers (Bogard et al. 2014), the mechanism for this process is not fully understood (summarized in Tang et al. 2016). Another controversy is whether methane produced in the oxic subsurface layers makes a large (Bogard et al. 2014) or small (Encinas Fernández et al. 2016) contribution to the amount of methane emitted from the lake surface. Finally, the possible production of CH4 under aerobic conditions (Karl et al. 2008; Damm et al. 2008) in marine systems has been debated. Although this process could explain the very low concentration (<4 nM) of CH4 in open and deep oceanic regions, it cannot explain the much larger concentration of CH4 (10–1000 nM) in shallow coastal areas, where CH4 undoubtedly comes from sediments (Borges et al. 2016, 2017).
Perspective 2: Roles of diverse methanotrophs (MOB) in food web dynamics
From a food-web perspective, MOB are key players in a new mode of pelagic–benthic coupling in lake ecosystems (Schindler and Scheuerell 2002). In the broadly accepted view of pelagic–benthic coupling in deep lakes, sedimentation of organic matter produced by pelagic production is the basal resource of benthic invertebrates and fish, which act as alternative resources of pelagic mobile predators such as zooplankton and fish. In the new mode of pelagic–benthic coupling in deep lakes, summer pelagic PP is transferred to the benthic layers, ultimately supporting the secondary production of pelagic zooplankton mediated by methane-based food webs along the water column.
Two aspects of this new mode of pelagic–benthic coupling need to be addressed. First, the contribution from the benthic (methanotrophs) to pelagic (zooplankton) habitats mainly occurs during winter. The coupling effect has a time delay because of the time required for sedimentation of particulate organic matter from the pelagic zone to the benthic zone and for subsequent biogeochemical processes in benthic habitats (sediment plus deep water column), which supply resources to zooplankton through methane-based food webs (Ho et al. 2016). Second, our microbial ecology studies (Kojima et al. 2014; Kobayashi et al. 2016) and other existing evidence (refs. in Fig. 3) indicate that the sources of biomass carbon from MOB to the microbial food web are more diverse than previously believed (Fig. 3). Methane is not always directly integrated into the microbial food web via assimilation of methane by MOB through aerobic methane oxidation. For example, carbon biomass of M. oxyfera (NC10) is assimilated by fixation of CO2, but not directly by carbon from methane (Rasigraf et al. 2014). Therefore, dominance of the M. oxyfera-like phylotype in anoxic layers of FTR (Kojima et al. 2014) implies that methane is completely respired as CO2. Some of the CO2 assimilated into the bacterial biomass could have originated from methane oxidation; thus, carbon from methanogenesis is only indirectly incorporated into MOB biomass and the microbial food web. In addition, the presence of Type I and II MOB (Methylocystis) in the deep layers (anaerobic or microaerobic condition) implies that fermentative reactions support their activities, which are coupled with the release of organic acids such as acetate (Vecherskaya et al. 2009; Kalyuzhnaya et al. 2013). These organic acids are substrates for the growth of some bacteria, including Methlocystis (Belova et al. 2011; Im et al. 2011), and are finally incorporated into the microbial food web (i.e., indirect incorporation of methane-originated carbon).
Schematic diagram illustrating our new conceptual model of pelagic–benthic coupling mediated by vertically structured diverse MOB groups. Some arrows are omitted for simplicity (e.g., release of CO2 from zooplankton). Distribution of microbes in benthic habitats (from microaerobic water column to anaerobic sediment) was not clearly separated in the FTR (FeiTsui Reservoir) project, although it is conceptually separable. Microbial members in sediment (fermenter and methanogen) were not targets of observation in this project. HB represents heterotrophic bacteria that utilize photosynthetic products (POM) and M. oxyfera represents Methylomirabilis oxyfera. Ref1: Belova et al. 2011; Im et al. 2011, Ref2: Borrel et al. (2011), Ref3: Vecherskaya et al. (2009) and Kalyuzhnaya et al. (2013), Ref4: Ettwig et al. (2010) and Rasigraf et al. (2014)
These diverse types of MOB and metabolic pathways from methane should be further explored to better understand the importance and mechanisms of methane-based food web dynamics. The next version of the dynamic model coupling methane processes and food web dynamics should incorporate these diverse processes. In addition, CO2 would be repeatedly recycled within the anaerobic food chain in the sediment and sediment–water column boundary (Fig. 3). Therefore, isotopic analysis of CO2 and MOB together with analysis of phospholipid fatty acids (e.g., Belova et al. 2011) will be necessary to elucidate the dominant reactions and estimate the timescale of interactions between CO2, methane, and MOB and, thus, the new pelagic–benthic coupling.
Perspective 3: Importance of studying underexplored tropical/subtropical lakes
Lakes in subtropical regions are highly dynamic in terms of their interannual climate variations and strength/frequency of disturbances. These factors are determinants of the vertical, seasonal, and interannual variations in microbes and biogeochemical processes. Such variations control the (1) balance of PP and aerobic respiration, (2) production and oxidation of methane, and (3) incorporation of methane-originated carbon into the pelagic food web (Figs. 1, 2, 3). Our multiple approaches to understanding methane dynamics targeted a subtropical reservoir (FTR) with an essentially monomictic pattern. As is the case in some reported subtropical lakes, FTR occasionally undergoes not only incomplete vertical mixing during winter, but also stronger and longer thermal stratification periods, resulting in profundal hypoxia (e.g., Sahoo and Schladow 2008; Yoshimizu et al. 2010). In contrast, intensive winter mixing can be observed during cold winters. These findings indicate that long-term studies of deep monomictic lakes and reservoirs at lower latitudes can reveal aspects of both meromictic and monomictic lakes.
Our study sheds new light on other important controlling factors of biogeochemical cycles, such as the disruption of stratification by heavy-rain events. Lower-latitude areas experience a higher frequency of heavy precipitation than mid- or high-latitude regions (Dai 2012). Under recent warming conditions, the frequency of heavy precipitation and the temperature of mid-latitude regions have been increasing and are predicted to increase further (e.g., Meehl et al. 2005). Knowledge of the response of methane dynamics in lower-latitude lakes to climate variations will make it possible to predict the future condition of mid-latitude lakes. Our case study showed that the effects of typhoons in summer on the ratio of PP to aerobic respiration and, thus, DO levels were depth-specific. Long-term comparative studies of other mero/monomictic lakes at the tropical/subtropical boundary (e.g., Okuda et al. 2017) will provide a more comprehensive understanding of mechanisms in lakes at a wide latitudinal scale in a changing world.
Change history
29 March 2018
The article “Integrating isotopic, microbial, and modeling approaches to understand methane dynamics in a frequently disturbed deep reservoir in Taiwan”, written by Masayuki Itoh, Hisaya Kojima, Pei-Chi Ho, Chun-Wei Chang, Tzong-Yueh Chen, Silver Sung-Yun Hsiao, Yuki Kobayashi, Megumu Fujibayashi, Shuh-Ji Kao, Chih-hao Hsieh, Manabu Fukui, Noboru Okuda, Takeshi Miki, Fuh-Kwo Shiah, was originally published electronically on the publisher’s internet portal (currently SpringerLink) on 18 September 2017 without open access.
References
Abril G, Guerin F, Richard S, Delmas R, Galy-Lacaux C, Gosse P, Tremblay A, Varfalvy L, Dos Santos MA, Matvienko B (2005) Carbon dioxide and methane emissions and the carbon budget of a 10-year old tropical reservoir (Petit Saut, French Guiana). Glob Biogeochem Cycles 19:Gb4007. doi:10.1029/2005gb002457
Bastviken D, Cole J, Pace M, Tranvik L (2004) Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob Biogeochem Cycles. doi:10.1029/2004gb002238
Bastviken D, Cole JJ, Pace ML, Van de Bogert MC (2008) Fates of methane from different lake habitats: connecting whole-lake budgets and methane emissions. J Geophys Res. doi:10.1029/2007jg000608
Bastviken D, Santoro AL, Marotta H, Pinho LQ, Calheiros DF, Crill P, Enrich-Prast A (2010) Methane emissions from Pantanal, South America, during the low water season: toward more comprehensive sampling. Environ Soc Technol 44:5450–5455. doi:10.1021/es1005048
Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A (2011) Freshwater methane emissions offset the continental carbon sink. Science 331:50. doi:10.1126/science.1196808
Bates TS, Kelly KC, Johnson JE, Gammon RH (1996) A reevaluation of the open ocean source of methane to the atmosphere. J Geophys Res Atmos 101:6953–6961. doi:10.1029/95JD03348
Belova SE, Baani M, Suzina NE, Bodelier PLE, Liesack W, Dedysh SN (2011) Acetate utilization as a survival strategy of peat-inhabiting Methylocystis spp. Environ Microbiol Rep 3:36–46. doi:10.1111/j.1758-2229.2010.00180.x
Bogard MJ, del Giorgio PA, Boutet L, Chaves MC, Prairie YT, Merante A, Derry AM (2014) Oxic water column methanogenesis as a major component of aquatic CH4 fluxes. Nat Commun 5:5350. doi:10.1038/ncomms6350
Borges AV, Abril G, Delille B, Descy JP, Darchambeau F (2011) Diffusive methane emissions to the atmosphere from Lake Kivu (Eastern Africa). J Geophys Res Biogeosci. doi:10.1029/2011jg001673
Borges AV, Darchambeau F, Teodoru CR, Marwick TR, Tamooh F, Geeraert N, Omengo FO, Guérin F, Lambert T, Morana C, Okuku E, Bouillon S (2015a) Globally significant greenhouse gas emissions from African inland waters. Nat Geosci 8:637–642. doi:10.1038/NGEO2486
Borges AV, Abril G, Darchambeau F, Teodoru CR, Deborde J, Vidal LO, Lambert T, Bouillon S (2015b) Divergent biophysical controls of aquatic CO2 and CH4 in the World’s two largest rivers. Sci Rep 5:15614. doi:10.1038/srep15614
Borges AV, Champenois W, Gypens N, Delille B, Harlay J (2016) Massive marine methane emissions from near-shore shallow coastal areas. Sci Rep 6:27908. doi:10.1038/srep27908
Borges AV, Speeckaert G, Champenois A, Scranton MI, Gypens N (2017) Productivity and temperature as drivers of seasonal and spatial variations of dissolved methane in the Southern Bight of the North Sea. Ecosystems. doi:10.1007/s10021-017-0171-7
Borrel G, Jézéquel D, Biderre-Petit C, Morel-Desrosiers N, Morel J-P, Peyret P, Fonty G, Lehours A-C (2011) Production and consumption of methane in freshwater lake ecosystems. Res Microbiol 162:832–847
Chang SP, Wen CG (1997) Changes in water quality in the newly impounded subtropical Feitsui Reservoirs, Taiwan. J Am Water Resour Assoc 33:343–357
Chanudet V, Descloux S, Harby A, Sundt H, Hansen BH, Brakstad O, Serca D, Guerin F (2011) Gross CO2 and methane emissions from the Nam Ngum and Nam Leuk sub-tropical reservoirs in Lao PDR. Sci Total Environ 409:5382–5391. doi:10.1016/j.scitotenv.2011.09.018
Ciais P, Sabine C, Govindasamy B, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni R, Piao S, Thornton P (2013) Chapter 6: carbon and other biogeochemical cycles. In: Stocker T, Qin D, Platner G-K (eds) Climate change 2013 the physical science basis. Cambridge University Press, Cambridge
Currie DJ, Kalff J (1984) A comparision of the abilities of freshwater algae and bacteria to acquire and retain phosphorus. Limnol Oceanogr 29:298–310
Dai A (2012) Increasing drought under global warming in observations and models. Nat Clim Change 3:52–58. doi:10.1038/nclimate1633
Damm E, Kiene RP, Schwarz J, Falck E, Dieckmann G (2008) Methane cycling in Arctic shelf water and its relationship with phytoplankton biomass and DMSP. Mar Chem 109:45–59
Deines P, Fink P (2011) The potential of methanotrophic bacteria to compensate for food quantity or food quality limitations in Daphnia. Aquat Microb Ecol 65:197–206
Deutzmann JS, Stief P, Brandes J, Schink B (2014) Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake. Proc Natl Acad Sci USA 111:18273–18278. doi:10.1073/pnas.1411617111
Eckert W, Conrad R (2007) Sulfide and methane evolution in the hypolimnion of a subtropical lake: a three-year study. Biogeochemistry 82:67–76. doi:10.1007/s10533-006-9053-3
Encinas Fernández J, Peeters F, Hofmann H (2016) On the methane paradox: transport from shallow water zones rather than in situ methanogenesis is the major source of CH4 in the open surface water of lakes. J Geophys Res Biogeosci 121:2717–2726. doi:10.1002/2016JG003586
Ettwig KF, van Alen T, van de Pas-Schoonen KT, Jetten MS, Strous M (2009) Enrichment and molecular detection of denitrifying methanotrophic bacteria of the NC10 phylum. Appl Environ Microbiol 75:3656–3662. doi:10.1128/AEM.00067-09
Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJ, van Alen T, Luesken F, Wu ML, van de Pas-Schoonen KT, Op den Camp HJ, Janssen-Megens EM, Francoijs KJ, Stunnenberg H, Weissenbach J, Jetten MS, Strous M (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548. doi:10.1038/nature08883
Fan C-W, Kao S-J (2008) Effects of climate events driven hydrodynamics on dissolved oxygen in a subtropical deep reservoir in Taiwan. Sci Total Environ 393:326–332. doi:10.1016/j.scitotenv.2007.12.018
Guérin F, Abril G (2007) Significance of pelagic aerobic methane oxidation in the methane and carbon budget of a tropical reservoir. J Geophys Res. doi:10.1029/2006jg000393
Hamdan LJ, Wickland KP (2016) Methane emissions from oceans, coasts, and freshwater habitats: new perspectives and feedbacks on climate. Limnol Oceanogr 61:S3–S12
Harrits SM, Hanson RS (1980) Stratification of aerobic methane-oxidizing organisms in Lake Mendota, Madison, Wisconsin. Limnol Oceanogr 25:412–421
Ho P-C, Okuda N, Miki T, Itoh M, Shiah F-K, Chang C-W, Hsiao SS-Y, Kao S-J, Fujibayashi M, Hsieh C-H (2016) Summer profundal hypoxia determines the coupling of methanotrophic production and the pelagic food web in a subtropical reservoir. Freshw Biol 61:1694–1706. doi:10.1111/fwb.12809
Huttunen JT, Alm J, Liikanen A, Juutinen S, Larmola T, Hammar T, Silvola J, Martikainen PJ (2003) Fluxes of methane, carbon dioxide and nitrous oxide in boreal lakes and potential anthropogenic effects on the aquatic greenhouse gas emissions. Chemosphere 52:609–621. doi:10.1016/S0045-6535(03)00243-1
Im J, Lee S-W, Yoon S, DiSpirito AA, Semrau JD (2011) Characterization of a novel facultative Methylocystis species capable of growth on methane, acetate and ethanol. Environ Microbiol Rep 3:174–181. doi:10.1111/j.1758-2229.2010.00204.x
Itoh M, Kobayashi Y, Chen T-Y, Tokida T, Fukui M, Kojima H, Miki T, Tayasu I, Shiah F-K, Okuda N (2015) Effect of interannual variation in winter vertical mixing on methane dynamics in a subtropical reservoir. J Geophys Res Biogeosci 120:1246–1261. doi:10.1002/2015JG002972
Jones RI, Grey J (2011) Biogenic methane in freshwater food webs. Freshw Biol 56:213–229. doi:10.1111/j.1365-2427.2010.02494.x
Ká Norði, Thamdrup B (2014) Nitrate-dependent anaerobic methane oxidation in a freshwater sediment. Geochim Cosmochim Acta 132:141–150. doi:10.1016/j.gca.2014.01.032
Kalyuzhnaya MG, Yang S, Rozova ON, Smalley NE, Clubb J, Lamb A, Gowda GAN, Raftery D, Fu Y, Bringel F, Vuilleumier S, Beck DAC, Trotsenko YA, Khmelenina VN, Lidstrom ME (2013) Highly efficient methane biocatalysis revealed in a methanotrophic bacterium. Nat Commun 4:2785. doi:10.1038/ncomms3785
Karl DM, Beversdorf L, Bjorkman KM, Church MJ, Martinez A, DeLong EF (2008) Aerobic production of methane in the sea. Nat Geosci 1:473–478
Kits KD, Klotz MG, Stein LY (2015) Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1. Environ Microbiol 17:3219–3232. doi:10.1111/1462-2920.12772
Kiyashko SI, Narita T, Wada E (2001) Contribution of methanotrophs to freshwater macroinvertebrates: evidence from stable isotope ratios. Aquat Microbiol Ecol 24:203–207
Kling GW, Kipphut GW, Miller MC (1992) The flux of CO2 and methane from lakes and rivers in Arctic Alaska. Hydrobiologia 240:23–36. doi:10.1007/Bf00013449
Ko C-Y, Lai C-C, Chen T-Y, Hsu H-H, Shiah F-K (2016) Typhoon effects on phytoplankton responses in a semi-closed freshwater ecosystem. Mar Freshw Res 67:546–555. doi:10.1071/MF14294
Ko C-Y, Lai C-C, Hsu H-H, Shiah F-K (2017) Decadal phytoplankton dynamics in response to episodic climatic disturbances in a subtropical deep freshwater ecosystem. Water Res 109:102–113. doi:10.1016/j.watres.2016.11.011
Kobayashi Y, Kojima H, Itoh M, Okuda N, Fukui M, Shiah F-K (2016) Abundance of planktonic methane-oxidizing bacteria in a subtropical reservoir. Plankton Benthos Res 11:144–146. doi:10.3800/pbr.11.144
Kojima H, Tokizawa R, Kogure K, Kobayashi Y, Itoh M, Shiah FK, Okuda N, Fukui M (2014) Community structure of planktonic methane-oxidizing bacteria in a subtropical reservoir characterized by dominance of phylotype closely related to nitrite reducer. Sci Rep 4:5728. doi:10.1038/srep05728
Laurion I, Vincent WF, MacIntyre S, Retamal L, Dupont C, Francus P, Pienitz R (2010) Variability in greenhouse gas emissions from permafrost thaw ponds. Limnol Oceanogr 55:115–133. doi:10.4319/lo.2010.55.1.0115
Lofton DD, Whalen SC, Hershey AE (2014) Effect of temperature on methane dynamics and evaluation of methane oxidation kinetics in shallow Arctic Alaskan lakes. Hydrobiologia 721:209–222. doi:10.1007/s10750-013-1663-x
Marotta H, Pinho L, Gudasz C, Bastviken D, Tranvik LJ, Enrich-Prast A (2014) Greenhouse gas production in low-latitude lake sediments responds strongly to warming. Nat Clim Change 4:467–470. doi:10.1038/Nclimate2222
Meehl GA, Arblaster JM, Tebaldi C (2005) Understanding future patterns of increased precipitation intensity in climate model simulations. Geophys Res Lett 32:L18719. doi:10.1029/2005gl023680
Michmerhuizen CM, Striegl RG, McDonald ME (1996) Potential methane emission from north-temperate lakes following ice melt. Limnol Oceanogr 41:985–991. doi:10.4319/lo.1996.41.5.0985
Morana C, Borges AV, Roland FAE, Darchambeau F, Descy J-P, Bouillion S (2015) Methanotrophy within the water column of a large meromictic tropical lake (Lake Kivu, East Africa). Biogeosciences 12:2077–2088
Murase J, Sakai Y, Kametani A, Sugimoto A (2005) Dynamics of methane in mesotrophic Lake Biwa, Japan. Ecol Res 20:377–385. doi:10.1007/s11284-005-0053-x
Okuda N, Sakai Y, Fukumori K, Yang S-M, Hsieh C-H, Shiah F-K (2017) Food web properties of the recently constructed, deep subtropical Fei-Tsui Reservoir in comparison with the ancient Lake Biwa. Freshw Biol. doi:10.1007/s10750-017-3258-4 (in print)
Ostrom NE, Russ ME, Field A, Piwinski L, Twiss MR, Carrick HJ (2005) Ratios of community respiration to photosynthesis and rates of primary production in Lake Erie via oxygen isotope techniques. J Great Lakes Res 31:138–153. doi:10.1016/S0380-1330(05)70310-5
Pacheco FS, Roland F, Downing JA (2013) Eutrophication reverses whole-lake carbon budgets. Inland Waters 4:41–48
Pasche N, Schmid M, Vazquez F, Schubert CJ, Wuest A, Kessler JD, Pack MA, Reeburgh WS, Burgmann H (2011) Methane sources and sinks in Lake Kivu. J Geophys Res Biogeosci 116:G03006. doi:10.1029/2011jg001690
Raghoebarsing AA, Pol A, van de Pas-Schoonen KT, Smolders AJ, Ettwig KF, Rijpstra WI, Schouten S, Damste JS, Op den Camp HJ, Jetten MS, Strous M (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918–921. doi:10.1038/nature04617
Rasigraf O, Kool DM, Jetten MSM, Sinninghe Damsté JS, Ettwig KF (2014) Autotrophic carbon dioxide fixation via the calvin-benson-bassham cycle by the denitrifying methanotroph “Candidatus Methylomirabilis oxyfera”. Appl Environ Microbiol 80:2451–2460. doi:10.1128/aem.04199-13
Rhee TS, Kettle AJ, Andreae MO (2009) Methane and nitrous oxide emissions from the ocean: a reassessment using basin-wide observations in the Atlantic. J Geophys Res Atmos. doi:10.1029/2008JD011662
Roland FAE, Darchambeau F, Morana C, Bouillon S, Borges AV (2017) Emission and oxidation of methane in a meromictic, eutrophic and temperate lake (Dendre, Belgium). Chemosphere 168:756–764
Rudd JWM (1980) Methane oxidation in Lake Tanganyika (East-Africa). Limnol Oceanogr 25:958–963
Sahoo GB, Schladow SG (2008) Impacts of climate change on lakes and reservoirs dynamics and restoration policies. Sustain Sci 3:189–199. doi:10.1007/s11625-008-0056-y
Schindler DE, Scheuerell MD (2002) Habitat coupling in lake ecosystems. Oikos 98:177–189. doi:10.1034/j.1600-0706.2002.980201.x
Shiah F-K, Chung S-W, Kao S-J, Gong G-C, Liu K-K (2000) Biological and hydrographical responses to tropical cyclones (typhoons) in the continental shelf of the Taiwan Strait. Cont Shelf Res 20:2029–2044. doi:10.1016/S0278-4343(00)00055-8
Taipale S, Kankaala P, Hämäläinen H, Jones RI (2009) Seasonal shifts in the diet of lake zooplankton revealed by phospholipid fatty acid analysis. Freshw Biol 54:90–104. doi:10.1111/j.1365-2427.2008.02094.x
Taipale S, Kankaala P, Hahn MW, Jones RI, Tiirola M (2011) Methane-oxidizing and photoautotrophic bacteria are major producers in a humic lake with a large anoxic hypolimnion. Aquat Microb Ecol 64:81–95
Tanaka Y, Tsuda R (1996) Daily fluctuations in thermal stratification, chlorophyll a, turbidity and dissolved oxygen concentration in Lake Biwa. Jpn J Limnol 57:377–393. doi:10.3739/rikusui.57.377
Tang KW, McGinnis DF, Frindte K, Brüchert V, Grossart H-P (2014) Paradox reconsidered: methane oversaturation in well-oxygenated lake waters. Limnol Oceanogr 59:275–284
Tang KW, McGinnis DF, Ionescu D, Grossart H-P (2016) Methane production in oxic lake waters potentially increases aquatic methane flux to air. Environ Sci Technol Lett 3:227–233. doi:10.1021/acs.estlett.6b00150
Thingstad TF, Hagström Å, Rassoulzadegan F (1997) Accumulation of degradable DOC in surface waters: is it caused by a malfunctioning microbial loop? Limnol Oceanogr 42:398–404. doi:10.4319/lo.1997.42.2.0398
Tranvik LJ, Downing JA, Cotner JB, Loiselle SA, Striegl RG, Ballatore TJ, Dillon P, Finlay K, Fortino K, Knoll LB, Kortelainen PL, Kutser T, Larsen S, Laurion I, Leech DM, McCallister SL, McKnight DM, Melack JM, Overholt E, Porter JA, Prairie Y, Renwick WH, Roland F, Sherman BS, Schindler DW, Sobek S, Tremblay A, Vanni MJ, Verschoor AM, von Wachenfeldt E, Weyhenmeyer GA (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr 54:2298–2314. doi:10.4319/lo.2009.54.6_part_2.2298
Tseng YF, Hsu TC, Chen YL, Kao SJ, Wu JT, Lu JC, Lai CC, Kuo HY, Lin CH, Yamamoto Y, Xiao T, Shiah FK (2010) Typhoon effects on DOC dynamics in a phosphate-limited reservoir. Aquat Microbial Ecol 60:247–260. doi:10.3354/ame01423
Tsuchiya K, Kuwahara VS, Hamasaki K, Tada Y, Ichikawa T, Yoshiki T, Nakajima R, Imai A, Shimode S, Toda T (2015) Typhoon-induced response of phytoplankton and bacteria in temperate coastal waters. Estuar Coast Shelf Sci 167(12):458–465. doi:10.1016/j.ecss.2015.10.026
Utsumi M, Nojiri Y, Nakamura T, Nozawa T, Otsuki A, Seki H (1998) Oxidation of dissolved methane in a eutrophic, shallow lake: lake Kasumigaura, Japan. Limnol Oceanogr 43:471–480
Vadstein O (2000) Heterotrophic, planktonic bacteria and cycling of phosphorus. Adv Microbiol 16:115–167
Vecherskaya M, Dijkema C, Saad HR, Stams AJM (2009) Microaerobic and anaerobic metabolism of a Methylocystis parvus strain isolated from a denitrifying bioreactor. Environ Microbiol Rep 1:442–449. doi:10.1111/j.1758-2229.2009.00069.x
Verburg P, Hecky RE, Kling H (2003) Ecological consequences of a century of warming in Lake Tanganyika. Science 301:505–507. doi:10.1126/science.1084846
Wetzel RG (1990) Land water interfaces: metabolic and limnological regulators. Verh Int Ver Limnol 24(6):24
Wilhelm S, Adrian R (2008) Impact of summer warming on the thermal characteristics of a polymictic lake and consequences for oxygen, nutrients and phytoplankton. Freshw Biol 53:226–237. doi:10.1111/j.1365-2427.2007.01887.x
Yao M, Henny C, Maresca JA (2016) Freshwater bacteria release methane as a by-product of phosphorus acquisition. Appl Environ Microbiol 82:6994–7003. doi:10.1128/aem.02399-16
Yoshimizu C, Yoshiyama K, Tayasu I, Koitabashi T, Nagata T (2010) Vulnerability of a large monomictic lake (Lake Biwa) to warm winter event. Limnology 11:233–239. doi:10.1007/s10201-009-0307-3
Acknowledgements
N. O. is supported by a JSPS Grant-in aid (no. 24405007 and 16H05774) and RIHN Project (D-06-14200119). This study was conducted under the Joint Research Program of the Institute of Low Temperature Science, Hokkaido University. T. M. was supported by MOST 103 - 2621 - M - 002 - 015 -.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The original version of this article was revised due to a retrospective Open Access.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Itoh, M., Kojima, H., Ho, PC. et al. Integrating isotopic, microbial, and modeling approaches to understand methane dynamics in a frequently disturbed deep reservoir in Taiwan. Ecol Res 32, 861–871 (2017). https://doi.org/10.1007/s11284-017-1502-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-017-1502-z