Abstract
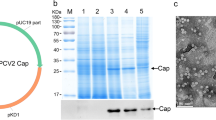
Porcine circovirus type 2 (PCV2) poses a genuine threat to pig industry. An effective vaccine production against the pandemic is desirable. The aim of this study was to construct recombination yeast strains with PCV2 Cap protein. We adopt to YeastFab Assembly method to synthesize transcriptional units in a single tube by piecing up promoter, open reading frame, and terminator in S. cerevisiae. Two yeast recombinants were successfully constructed using GPD and TEF2 promoters, respectively, to express PCV2 by secreting Cap protein in vitro. Electronic microscope observation demonstrated that the yeast-derived PCV2 Cap protein could self-assembles into 18-nm-diameter virus-like particles (VLPs). The yield of two different recombination yeasts containing GPD and TEF2 promoters were 12, 25 μg/ml, respectively. Our results showed that it is feasible to use S. cerevisiae as a safe and simple system to produce PCV2 virus-like particles. This indicated that there is possibility of obtaining PCV2 VLP vaccine by homologous recombination in yeast genome, and Cap protein was secreted into the cultural supernatant which can be used as a potential oral vaccine to protect pigs from PCV2-infection.






Similar content being viewed by others
References
R. Patterson et al., Oral application of freeze-dried yeast particles expressing the PCV2b Cap protein on their surface induce protection to subsequent PCV2b challenge in vivo. Vaccine 33(46), 6199–6205 (2015)
P. Nawagitgul et al., Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 81(Pt 9), 2281–2287 (2000)
Y. Hu et al., Evidence of natural co-infection with PCV2b subtypes in vivo. Arch. Virol. 162(7), 2015–2020 (2017)
H. Vigre et al., Spatial and temporal patterns of pig herds diagnosed with postweaning multisystemic wasting syndrome (PMWS) during the first two years of its occurrence in Denmark. Vet. Microbiol. 110(1–2), 17–26 (2005)
Y.F. Fu et al., Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci Rep 7(1), 8676 (2017)
S. Pouyanfard, M. Muller, Human papillomavirus first and second generation vaccines-current status and future directions. Biol. Chem. 398(8), 871–889 (2017)
H.J. Kim, H.J. Kim, Yeast as an expression system for producing virus-like particles: what factors do we need to consider? Lett. Appl. Microbiol. 64(2), 111–123 (2017)
V. Manolova et al., Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 38(5), 1404–1413 (2008)
S.A. Bucarey et al., The optimized capsid gene of porcine circovirus type 2 expressed in yeast forms virus-like particles and elicits antibody responses in mice fed with recombinant yeast extracts. Vaccine 27(42), 5781–5790 (2009)
J. Nainys et al., Generation in yeast of recombinant virus-like particles of porcine circovirus type 2 capsid protein and their use for a serologic assay and development of monoclonal antibodies. BMC Biotechnol. 14, 100 (2014)
G. Franzo et al., Revisiting the taxonomical classification of porcine circovirus type 2 (PCV2): still a real challenge. Virol J 12, 131 (2015)
B. Davies et al., Diagnostic phylogenetics reveals a new porcine circovirus 2 cluster. Virus Res. 217, 32–37 (2016)
Y. Guo et al., YeastFab: the design and construction of standard biological parts for metabolic engineering in Saccharomyces cerevisiae. Nucleic Acids Res. 43(13), e88 (2015)
T. Wu et al., Hepatitis E vaccine development: a 14 year odyssey. Hum Vaccin Immunother 8(6), 823–827 (2012)
I.H. Frazer, Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 4(1), 46–54 (2004)
W. Li et al., Genetic analysis of porcine circovirus type 2 (PCV2) strains isolated between 2001 and 2009: genotype PCV2b predominate in postweaning multisystemic wasting syndrome occurrences in eastern China. Virus Genes 40(2), 244–251 (2010)
Q. Hou et al., Nuclear localization signal regulates porcine circovirus type 2 capsid protein nuclear export through phosphorylation. Virus Res. 246, 12–22 (2017)
J. Lopez-Vidal et al., Improved production efficiency of virus-like particles by the baculovirus expression vector system. PLoS ONE 10(10), e0140039 (2015)
A.L. Hamel, L.L. Lin, G.P. Nayar, Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 72(6), 5262–5267 (1998)
H. Fan et al., Immunogenicity of empty capsids of porcine circovirus type 2 produced in insect cells. Vet. Res. Commun. 31(4), 487–496 (2007)
S.L. Zhai et al., Complete genome characterization and phylogenetic analysis of three distinct buffalo-origin PCV2 isolates from China. Infect Genet Evol 28, 278–282 (2014)
M.A. Ssemadaali, M. Ilha, S. Ramamoorthy, Genetic diversity of porcine circovirus type 2 and implications for detection and control. Res. Vet. Sci. 103, 179–186 (2015)
C.T. Xiao, P.G. Halbur, T. Opriessnig, Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 96(Pt 7), 1830–1841 (2015)
F. Pitoiset, T. Vazquez, B. Bellier, Enveloped virus-like particle platforms: vaccines of the future? Expert Rev Vaccines 14(7), 913–915 (2015)
P. Nawagitgul et al., Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin. Diagn. Lab. Immunol. 9(1), 33–40 (2002)
J.Y. Zhou et al., In vitro expression, monoclonal antibody and bioactivity for capsid protein of porcine circovirus type II without nuclear localization signal. J. Biotechnol. 118(2), 201–211 (2005)
Z. Marcekova et al., Heterologous expression of full-length capsid protein of porcine circovirus 2 in Escherichia coli and its potential use for detection of antibodies. J. Virol. Methods 162(1–2), 133–141 (2009)
K. Wang et al., Expression of the capsid protein of porcine circovirus type 2 in Lactococcus lactis for oral vaccination. J. Virol. Methods 150(1–2), 1–6 (2008)
M. Trundova, V. Celer, Expression of porcine circovirus 2 ORF2 gene requires codon optimized E. coli cells. Virus Genes 34(2), 199–204 (2007)
P.C. Wu et al., Efficient expression and purification of porcine circovirus type 2 virus-like particles in Escherichia coli. J. Biotechnol. 220, 78–85 (2016)
A. Cid-Arregui, V. Juarez, H. zur Hausen, A synthetic E7 gene of human papillomavirus type 16 that yields enhanced expression of the protein in mammalian cells and is useful for DNA immunization studies. J. Virol. 77(8), 4928–4937 (2003)
C. Engler, R. Kandzia, S. Marillonnet, A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3(11), e3647 (2008)
Acknowledgements
We thank prof. Junbiao Dai of Tsinghua University for providing us the YeastFab related materials and methods. This work was supported by the National Natural Science Foundation of Youth Science Foundation in China (31400064), and the Underprop Project of Tianjin Science and Technology Committee in China (16YFZCNC00640).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Jinhai Huang. Performed the experiments: Pei Chen, Tian Gao, Na Chang, Chengxue Zhao, Dong Lu, and Lilin Zhang. Analyzed the data: Lilin Zhang and Pei Chen. Contributed reagents/materials/analysis tools: Jinhai Huang. Wrote the paper: Pei Chen and Lilin Zhang.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
This article does not include any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in this article.
Additional information
Edited by Zhen F. Fu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, P., Zhang, L., Chang, N. et al. Preparation of virus-like particles for porcine circovirus type 2 by YeastFab Assembly. Virus Genes 54, 246–255 (2018). https://doi.org/10.1007/s11262-018-1537-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-018-1537-4