Abstract
Urban areas are beginning to be recognized as having potential for biodiversity conservation because of the relatively high richness of some taxa. However, little is known about what functional groups of organisms constitute this richness. We investigated biodiversity patterns in an abandoned quarry using the Rapid Biodiversity Assessment method based on insect groups according to their trophic guilds. We assessed the value of semi-natural and ruderal habitats. The ruderal sites were characterized by a higher diversity and abundance of phytophagous beetles and, unexpectedly, of parasitoids and predatory beetles, whereas the reverse was true for Hemiptera and Aculeata. Patterns of α-diversity were impacted by different factors than β-diversity: these primarily acted in opposite directions, depending on the habitat type. Species richness was positively related to the woody surroundings of habitat patches on semi-natural sites, but negatively related on ruderal sites. The Dominance index was negatively affected by human impact. Insect assemblages were diversified by taller vegetation, higher nutrient content, lesser human impact and a lower level of insolation in the grasslands with ruderal vegetation than in the semi-natural grasslands. A particular habitat type may constitute a source for some insect groups but a sink for others. Ruderal habitats utilized as a substitute for loss of the semi-natural vegetation are essential for the preservation of insect functional diversity and are suitable for vulnerable groups such as Parasitica. Post-industrial areas with a habitat mosaic of semi-natural and ruderal sites may enrich biodiversity in the urban landscape.
Similar content being viewed by others
Introduction
Pressure on the natural environment is increasing globally (Obrist and Duelli 2010). Urbanization is expanding, particularly in some developing countries, where high-biodiversity sources are endangered (Ricketts and Imhoff 2003; Lee 2007). Populations of animals have become more prone to extinction (Hanski 1999). Many studies have revealed biodiversity losses caused by the expansion of urban space, changes in land use or invasions of alien species (Roy et al. 2003; Antrop and Van Eetvelde 2000; Nakamura et al. 2007; Aviron et al. 2009; Chace and Walsh 2006; Manning et al. 2016). Habitat fragmentation, the development of road networks leading to greater volumes of traffic is reducing suitable habitat area and increasing habitat patch isolation, road kill and core habitat deterioration (Fahrig 2003; Skórka et al. 2013). Invasive species such as goldenrods in Europe are displacing natural vegetation in invaded habitats (Moroń et al. 2009). Habitat suitability for wildlife is also decreasing as a result of agricultural intensification, landscape homogenization, the abandonment of traditional extensive grassland management and building development, e.g. on xerothermic grasslands (Skórka et al. 2007; Concepción et al. 2008). All these environmental changes are reducing ecosystem complexity, especially in urban areas.
Simultaneously, natural and semi-natural habitats have disappeared from many regions of Europe (WallisDeVries et al. 2002). In fragmented landscapes, species assemblages of insects and birds often occur in a nested structure: some habitats with lower species richness are subsets of others, including a greater variety of species (Fischer and Lindenmayer 2005; Schouten et al. 2007; Driscoll 2008). In a highly fragmented landscape with artificial greenery, the species assemblages of birds and butterflies tend to be more homogeneous within a given set of environmental conditions, and the fauna is usually poorer (Yan Chong et al. 2014). Finally, understanding how human-altered habitats may be valuable for biodiversity conservation has become a key problem of conservation biology and wildlife ecology.
Dispersion and immigration are essential processes for driving high species richness in urban ecosystems (Natuhara et al. 1999; Obrist and Duelli 2010; Lizée et al. 2012). Low habitat patch connectivity in urban areas hampers metapopulation dynamics, making dispersion risky and ineffective, especially for less mobile organisms such as plants or ground arthropods (Gilbert 1989; Denys and Schmidt 1998; Gonthier et al. 2014). Natural and semi-natural vegetation related to habitat heterogeneity are eminently suitable for maintaining ecosystem complexity and high biodiversity in small, scattered habitat patches in urban areas (Sattler et al. 2010). Some studies report that arthropod assemblages in a fragmented urban landscape tend to have a greater share of small species, capable of flight, than suburban areas, which are characterized rather by a high abundance of large, non-flying specialist species (Niemelä et al. 2002; Niemelä and Kotze 2009). In an urban landscape, the diversity of carabid beetles and parasitoids decreases, while the diversity of phytophagous beetles increases in comparison with rural areas (Denys and Schmidt 1998; Sattler et al. 2010). According to another study, however, urban dry meadows support a high carabid diversity and are an important habitat for xerophilic, granivorous and autumn-breeding species of carabid beetles (Venn et al. 2013). Therefore, the monitoring of insect assemblages while focusing on their trophic guilds may help to support conservation actions and provide deeper insight into the conditions of urban ecosystems (Angold et al. 2006; Helden et al. 2012).
Insects are key indicators of environmental changes, as they play an important role in nutrient cycling, decomposition, soil aeration and pollination; hence, they can be used for investigating the value of urban areas for biodiversity conservation (Maes and Van Dyck 2005; Thompson and McLachlan 2007; Clarke et al. 2008; Hunter and Hunter 2008; Vonshak et al. 2009). Insects are intensively affected by landscape structure (McIntyre 2000; Matteson et al. 2013); their abundance and diversity depend on the chemical, physical and ecological properties of the environment (Witmer et al. 2003). As particular trophic groups of insects have different functions in nature (e.g. predators, parasitoids, phytophages, pollinators), patterns of insect diversity provide useful information for understanding ecosystem functioning and local environmental changes, especially in urban areas, which used to be regarded as unattractive for both mainstream ecological research and biodiversity conservation (cf. Helden and Leather 2004; Snep et al. 2006; Luck and Smallbone 2010). However, urban areas may sustain species-rich ecosystems (Luck and Smallbone 2010). These include post-industrial sites, generally referred to as brownfield sites, such as abandoned quarries.
Quarrying gives rise to important environmental changes because it destroys vegetation and soil, thereby modifying the entire landscape. While the extraction of minerals has a serious negative impact on biodiversity, an abandoned quarry can subsequently enhance biodiversity by functioning as a refuge for many plant and animal assemblages, even rare and endangered species. The conservation potential of quarry areas has been reported for both arthropods and plants (see Tropek et al. 2010; Bétard 2013 with references). In our study, we investigated the abandoned Zakrzówek Quarry, established in an outcrop of Jurassic limestone. The quarry is covered and surrounded by pine and deciduous forest and a mosaic of semi-natural grassland, degraded wet meadows and grassland with ruderal vegetation. We classified the latter two habitats as ruderal vegetation. Thus, we examined open habitats, i.e. patches of semi-natural grassland, and patches of ruderal grassland that come into being spontaneously after disturbance.
Our study aimed to identify the key features of quarry areas that support insect functional diversity and to test the suitability of a simplified method of biodiversity assessment based on morphospecies and insect functional groups, according to their trophic guilds. We hypothesized that 1) in post-industrial areas such as an abandoned quarry, a mosaic of semi-natural and ruderal habitats supports the conservation of insect diversity better than a single group of habitats does; i.e. ruderal habitats also have an important conservation value; 2) the insect diversity responds in different ways to environmental characteristics with regard to diversity level (α- and β-diversity) and habitat types; 3) semi-natural habitats are characterized by a high diversity of pollinator and parasitoid species owing to the great variety of ecological niches, the wide range of plant species and warmer conditions; in contrast, the insect assemblages of ruderal habitats contain a larger percentage of phytophagous species because primary productivity in these habitats may be higher (cf. Venn et al. 2013).
Methods
Study area
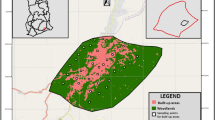
The study was carried out in 2008 within a grassland complex situated in the abandoned calcareous Zakrzówek Quarry near the Wawel Royal Castle in Kraków, southern Poland. The quarry, isolated and surrounded by urban infrastructure, had been excavated in an outcrop of Jurassic limestone (Oxfordian). The quarry floor was flooded. The quarry itself and its immediate surroundings were covered by grassland, as well as pine and deciduous forests (Fig. 1). The grasslands in the quarry formed a habitat mosaic of xerothermic calcareous grassland, wet and lowland hay meadows, degraded meadows with reeds, and grassland with ruderal vegetation. The xerothermic grasslands consisted of vegetation of the Festuco-Brometea class. The hay meadows were covered with vegetation from the orders Arrhenatheretalia and occasionally Molinietalia, and the grasslands with ruderal vegetation were represented by the community of tansy and mugworts (Artemisio-Tanacetum vulgaris) and other ruderal communities (Dubiel 2005). We selected 19 sites: 5 xerothermic grasslands, 2 thermophilous false oat-grass meadows (Arrhenatheretum elatioris), 5 false oat-grass meadows, 5 meadows with ruderal vegetation, and 2 wet meadows with reeds (Fig. 1). All these grasslands were relatively small – less than 1 ha in area. Finally, we distinguished two main types of environment: semi-natural meadows (the hay meadows, thermophilous meadows and xerothermic grasslands) and ruderal degraded sites (the ruderal plant communities and the wet meadows with reeds). We took all the ruderal sites situated within the quarry area into consideration.
Field study
Each site was visited three times: on 27 and 28 May, and on 3 June. The study was conducted between 11:00 and 16:00 h in sunny and windless weather with temperatures of 20–23 °C. At all sites three independent observers, each person at different times, moved through the grassland and sampled the insects with 75 strokes of a sweep net through the vegetation (a total of 225 strokes per visit). The observer started at random and made 25 St in one direction, then continued sampling in another direction making another 25 strokes. His path formed a broken line with obtuse turning angles.
The sampled material was sorted. The individual insects, separately for each site, were divided into morphospecies using the Rapid Biodiversity Assessment method. Diptera and juvenile forms were excluded from the investigation as they generate a systematic bias of the estimated biodiversity (see Oliver and Beattie 1995; Duelli et al. 1999). All morphospecies were identified as belonging to one ecological functional group of insects: Aculeata, Formicidae, Hemiptera, Lepidoptera, Orthoptera, Parasitica (Hymenoptera), Symphyta, herbivorous beetles, predatory beetles, omnivorous beetles, and other insects. The ecological functions of some insect orders, e.g. Coleoptera and Hymenoptera, differ within a taxonomic group, so these groups were investigated in more detail. On the other hand, the great majority of Lepidoptera and Symphyta, for example, are phytophagous, so it was sufficient to treat them at the order level.
The following α-diversity indices were calculated: the total number of morphospecies at each site and the total number of individuals at each site, i.e. the total numbers of individuals and morphospecies collected during all three visits. The following simplified measures of β-diversity were adopted: a presence-absence matrix of particular insect functional groups per site, the number of morphospecies belonging to particular insect functional groups per site (e.g. site 1–10 morphospecies belonging to Aculeata, 5 morphospecies belonging to Formicidae) and the number of individuals belonging to particular insect functional groups listed for each site.
We recorded a number of environmental variables using a simple scale in all the grasslands: vegetation height (1–4); general intensity of human impact (including mowing and trampling) (0–2); insolation (0–2); shelter from wind, expressed as the percentage of patch perimeter protected by forest or shrubs (0 – < 30% of the surroundings is woodland, 1 – > 30–60%, 2 – > 60%); calcareous substrate (0 – clayey soil or limestone debris not visible on the ground, 1 – limestone or limestone debris visible on the ground); nitrogen content as reflected by the relative proportions of Urtica dioica L. and other plants associated with high soil nitrogen levels (0–1); trampling (0 – no trampling, 1 – visible signs of constant trampling, e.g. paths, flattened vegetation, a high proportion of Lolium perenne L. in some places); mowing (0 – grassland not mown for several years, 1 – grassland mown once a year or mown in the year of our study).
Data handling and statistical analysis
We used principal components analysis PCA (Lepš and Šmilauer 2003) to summarize the eight aforementioned indices of grassland quality and to avoid collinearity problems in the analyses of biodiversity patterns, e.g. in the general linear model GLM analyses. PCA was performed by constructing a correlation matrix of the data. The relevance of the first PC1, second PC2 and third PC3 ordination axes was determined by PCA, i.e. the variance of the data was described significantly by the axes. Only those axes were considered in the subsequent analyses. The PCA scores of variables for each axis are shown in Table 1.
To obtain additional information regarding the α-diversity pattern, we calculated three further diversity measures: the Margalef richness index – a general measure of species richness; the Dominance D index – a measure of the dominance of some species in a species assemblage (range 0–1); the Evenness index – a measure of species assemblage evenness, i.e. the relatively equal representation of all species in the species assemblage (range 0–1) (for more details, see Harper 1999).
The first analysis using GLM modelling for the above-mentioned variables, i.e. the total number of morphospecies with a Poisson distribution, the total number of individuals with a negative binomial distribution and the last three diversity indices with a Gaussian distribution of the dependent variable, aimed to identify factors affecting α-diversity at the sites investigated. Separately for the five response variables (see above), we built global models that contained the following predictors: habitat type (semi-natural and ruderal), PC1, PC2, PC3 and their interaction terms with habitat type. Using Akaike information criteria with a correction for small sample sizes (AICc), we ranked models that were linear combinations of the predictor variables (Burnham and Anderson 2002). We ranked all the models built according to their ΔAICc values, where ΔAICc is the difference between a given model and the one with the lowest AICc. We defined as supported all the models with Akaike weight > 0.01, where the Akaike weight reflects the probability that a given model is the best one (Hurvich and Tsai 1989; Burnham and Anderson 2002). Next, we applied a model-averaging procedure using the parameter estimate and Akaike weight of each model. We also assessed the relative importance of each independent variable by calculating the cumulative Akaike weights of models containing a particular predictor (Burnham and Anderson 2002). The final models contained only significant interaction terms. Additionally, we checked for spatial autocorrelation in all species richness indices with Moran’s I at two distance classes using SAM software (Rangel et al. 2010).
The second analysis set out to assess whether there was a functional group turnover and a nested subset pattern of β-diversity. To test the first pattern of β-diversity, we performed a nonmetric multidimensional scaling NMS in four dimensions (Kindt and Coe 2005) for the presence-absence of insect functional groups, the number of morphospecies in a particular functional group and the number of individuals belonging to each particular functional group. Next, to evaluate the impact of habitat types on the variables tested, we applied PERMANOVAs based on Raup-Crick distances for presence-absence and on Bray-Curtis distances for the other two dependent variables (Anderson 2001; Quinn and Keough 2002). We carried out SIMPER analysis (Hammer et al. 2001) to describe in detail the average dissimilarity of insect functional group assemblages for semi-natural habitats and ruderal habitats. In the last step, we performed distance-based Redundancy Analyses db-RDA (Kindt and Coe 2005) using NMS data to test the influence of environmental variables (PC1-PC3) on functional group turnover. Moreover, to test whether the differences in dissimilarity were spatially autocorrelated, i.e. spatial autocorrelation in the assemblage composition, we performed Mantel tests with 999 permutations.
To check whether functional groups of insects recorded in particular grasslands represent nested subsets of the same pool of insects, the presence-absence matrices of functional group occurrence were analysed using the nestedness calculator of Atmar and Patterson (1995). This software compares the unexpected presence/absence of a functional insect group at particular sites with the null model of maximum disorder of functional group occurrence at all the sites investigated (Atmar and Patterson 1993). It is likely that the observed patterns are not true presence-absence ones, but they may reflect the poor representation of some insect groups.
The GLM modelling and Mantel test were performed in R 3.4.3 software and MuMIn, MASS and vegan packages (R Core Team 2017). The PCA, NMS and db-RDA ordinations were carried out with Canoco for Windows 4.5 (Lepš and Šmilauer 2003). The PERMANOVA, SIMPER analyses and α-diversity indices were calculated in PAST 3.01 (Hammer et al. 2001).
Results
We collected a total of 4012 insects. We recorded the highest number of individual insects (479) on xerothermic grassland 5, and the lowest number (87) at ruderal site 3 and hay meadow 3. The number of morphospecies ranged from 41 at ruderal site 5 to 77 at ruderal site 4.
Factors affecting α-diversity
The interpretation of the model selection and parameter averaging procedures is based on the PCA axis. The results of the analysis indicated that the number of morphospecies was governed by the PC2 – habitat type interaction term (Table 2). The number of morphospecies at the sites with ruderal vegetation was negatively related to the degree of shelter of the grasslands from the wind, while species richness was higher at the sites with semi-natural vegetation that were surrounded by forest or shrubs (Tables 1 and 2). On the other hand, we found no factor affecting the total number of individuals (Table 2).
The Dominance D index was negatively affected by PC3, which indicated increasing human influence and the absence of a calcareous substrate (Tables 1 and 3), i.e. the Dominance D index was higher at sites on a calcareous substrate (Table 1). The Evenness index was positively impacted by PC3 (Table 3): species evenness was therefore greater at the sites affected by human impact and at the sites situated on a non-calcareous substrate (Table 1). The Margalef richness index was influenced by the PC2 – habitat type interaction term (Table 3): at sites with ruderal vegetation, this index was negatively related to the degree of shelter from the wind, but took a higher value on sites with semi-natural vegetation that were surrounded by forest or shrubs (Table 1).
We found the following indices to be spatially independent: the number of morphospecies (1 class P = 0.579, 2 class P = 0.890), the Dominance D index (1 class P = 0.108, 2 class P = 0.259), the Margalef index (1 class P = 0.413, 2 class P = 0.533). In contrast, the number of individuals was slightly negatively autocorrelated at a more distant class (1 class P = 0.233, 2 class P = 0.038), and the Evenness index was negatively autocorrelated at a close distance class (1 class P < 0.001, 2 class P = 0.138).
β-diversity of insect functional groups on grasslands with semi-natural and ruderal vegetation
PERMANOVA revealed significant differences between grasslands with semi-natural and ruderal vegetation in species richness (F = 3.149, P = 0.007) and the total number of individuals (F = 2.804, P = 0.031) belonging to insect functional groups, whereas no difference was found in the presence-absence pattern of the functional groups (F = 1.89, P = 0.223). For both the number of morphospecies and the number of individuals of insect functional groups, the difference was related mainly to herbivorous beetles, Hemiptera, Parasitica and Aculeata (Table 4, Fig. 2). Hemiptera and Aculeata were more abundant and were represented by higher numbers of morphospecies in semi-natural grasslands, while herbivorous beetles and Parasitica (Hymenoptera) were more abundant and were represented by higher numbers of morphospecies in grasslands with ruderal vegetation (Table 4, Fig. 2).
For the presence-absence of functional groups, the first and second ordination axes of the db-RDA explained 23.5% of the variation in group composition, 89.8% of which was explained by the group-environmental relationship (significance of all axes F = 1.767, P = 0.073). For the number of species belonging to functional groups, the first and second ordination axes of the db-RDA explained 25.0% of the variation in group composition, 96.5% of which was explained by the group-environmental relationship (significance of all axes F = 1.749, P = 0.075). For the total number of individuals belonging to functional groups, the first and second ordination axes of the db-RDA explained 26.8% of the variation in group composition, 94.5% of which was explained by the group-environmental relationship (significance of all axes F = 1.984, P = 0.046).
In all cases, the insect functional groups were affected predominantly by PC1. The assemblages of functional groups were governed by taller vegetation, greater nutrient contamination, lower human impact (mowing and trampling), and lower insolation in grasslands with ruderal vegetation in comparison with semi-natural grasslands (Table 1, Fig. 2). The effects indicated by PC1 were significant with regard to the number of individuals, while in the case of presence-absence and the number of morphospecies, they were close to the significance level (see above). All the β-diversity indices were significantly but moderately autocorrelated: the presence-absence of an insect functional group (r = 0.160, P = 0.034), the number of morphospecies (r = 0.213, P = 0.012) and the number of individuals belonging to particular functional groups (r = 0.326, P = 0.002). Furthermore, the presence-absence pattern of functional groups revealed significant nestedness (T observed = 18.9° < T mean = 42.7° ± 9.27°; P = 0.002, Fig. 3).
Nested subset pattern of insect functional group composition. Nestedness matrix; columns represent insect groups, rows represent sites. The numbers under the matrix denote the number of insect functional group occurrences, while the numbers next to the rows represent the number of insect groups at a particular site. The insect group codes are denoted by Roman numerals, and the abbreviations represent site codes. The system temperature is marked as a horizontal line. I: Formicidae, Hemiptera, predatory beetles, herbivorous beetles; II: Parasitica; III: Other; IV: Symphyta; V: Aculeata; VI: omnivorous beetles; VII: Orthoptera; VIII: Lepidoptera. Xero. grassland – xerothermic grassland; Therm. meadow – thermophilous meadow; Meadow – hay meadow; Rud. grassland – Ruderal grassland; Degr. meadow – degraded wet meadow
Discussion
We found that ruderal sites were characterized by a higher diversity and abundance of phytophagous beetles and, unexpectedly, of parasitoids and predatory beetles, whereas Hemiptera and Aculeata were better represented in semi-natural grasslands. Our findings also demonstrated that the factors influencing the diversity patterns at the abandoned quarry could operate in different directions in the semi-natural grasslands and the sites with ruderal vegetation. This poses different challenges for biodiversity conservation in an urban landscape. This study underlines the importance of a habitat mosaic of semi-natural grasslands and ruderal grasslands for the preservation of insect functional diversity in urban areas. Isolated and surrounded by urban infrastructure, this abandoned quarry, which contains just such a habitat mosaic, appears to be a biodiversity hot spot in the city centre (cf. Kudłek et al. 2005).
It is probable that these two habitat types – semi-natural grassland and ruderal grassland – function as both a source and a sink, but for different insect functional groups (Pulliam and Danielson 1999). The positive, moderate spatial autocorrelation (Rangel et al. 2010) of the β-diversity suggests the existence of such source-sink dynamics. The insect assemblages from the semi-natural and ruderal habitats appear to complement each other. Semi-natural grasslands, especially xerothermic ones, support a variety of flowering plants (WallisDeVries et al. 2002) and are the most appropriate habitat for pollinators such as solitary bees and bumblebees as well as thermophilous Hemiptera, among which Cercopis vulnerata (Rossi, 1807) and Graphosoma lineatum (Linnaeus, 1758) were the most common species on the xerothermic grassland and thermophilous false oat-grass meadow. For these insect groups, the semi-natural meadow is a source because of its high diversity of plant species. On the other hand, grasslands with ruderal vegetation provide good host plant resources for phytophagous beetles, Orthoptera and Symphyta: polyphagous species in particular have excellent living conditions at the ruderal sites. Quite unexpectedly, however, the largest diversity of parasitoids recorded at ruderal sites contradicts the results of previous studies (Denys and Schmidt 1998; Sattler et al. 2010).
Although the woodland and shrubs surrounding the grassland positively influenced species richness in the semi-natural habitats, they had a negative impact in the ruderal habitats. Such results suggest that the woody surroundings of semi-natural grassland are important for specialist species, especially butterflies, for which they improve habitat quality (Akeboshi et al. 2015; Kalarus and Nowicki 2015 with references). For overgrowing ruderal habitats, characterized by tall vegetation (for example, Artemisia vulgaris L.), the woody surroundings of such patches appears to impoverish habitat quality, as trees and shrubs can lead to the loss the open, grassy character of such sites. The Dominance and Evenness indices are strongly related to disturbances such as trampling and mowing; species evenness is improved by these disturbances. The findings reveal the importance of minimal disturbances in maintaining the complexity of species assemblages in both habitat types. Parasitoids are known to be vulnerable to deep disturbance and habitat deterioration (cf. Kruess and Tscharntke 1994; Tscharntke et al. 2007; Řehounková et al. 2016). We conclude that ruderal habitats experiencing slight and rare disturbances, sufficient to maintain the habitat in its early successional stages, will support the development of parasitoids there. On the other hand, Hemiptera and Aculeata benefit from more frequent disturbances, such as mowing and constant trampling.
In our study, the β-diversity of insect functional groups in the abandoned quarry was affected by a different set of factors than α-diversity. The proportions and abundances of insect functional groups at the Zakrzówek Quarry were related mostly to the high nutrient content in the ground, the taller vegetation and the lesser human impact at ruderal sites, whereas the reverse was true for semi-natural sites. A high nutrient content can stimulate the production of plant biomass, leading to the successful development of phytophagous insects (Borer et al. 2012). The ready availability of phytophagous prey and also the low level of disturbances such as mowing in ruderal habitats appear to support a higher diversity of predatory beetles, including autumn breeders that demand a more stable habitat (Venn et al. 2013). This suggests that ruderal sites improve ecosystem services in the urban landscape (cf. Tilman et al. 2006), especially since a recent study has indicated that other insect groups such as Diptera, Coleoptera or Formicidae are as effective as bees in providing pollination services. Hence, insects other than bees perform a valuable service, offering a potential insurance against bee declines (Rader et al. 2016).
Altogether, such partially disturbed, overgrowing ruderal sites appear to support a high insect functional diversity in post-industrial areas and should not be neglected in conservation schemes.
Our results suggest that city centre ruderal habitats act in much the same way as suburban habitats or highly altered habitats with artificial greenery in biodiversity conservation. If they are utilized as a substitute for the loss of semi-natural vegetation, then they can improve overall urban diversity (cf. Venn et al. 2013; Yan Chong et al. 2014). Some studies imply that, apart from semi-natural and ruderal habitats, artificial and cultivated greenery may prevent biodiversity loss. For example, garden areas and artificial greenery have great value for maintaining biodiversity in the urban landscape (Galluzzi et al. 2010; Goddard et al. 2010), although another study has clearly demonstrated that they do not support complex species assemblages (Yan Chong et al. 2014). All these studies were conducted in other climatic zones. A study from England suggests that native tree species support a higher insect diversity than non-native species do (Helden et al. 2012). We have hypothesized that in the temperate climate zone, artificial greenery is not really appropriate for the conservation of biodiversity and the reclamation of post-mining areas, as it appears to have a simpler species composition and structure compared to both semi-natural and ruderal vegetation, which experiences spontaneous succession (cf. Tropek et al. 2010; Yan Chong et al. 2014). On the other hand, the value of ruderal vegetation and attendant types of degraded urban grasslands may decrease if nutrient levels increase substantially, ultimately leading to the dominance of nitrophilous plant species (Venn et al. 2013). In our study system, the predatory carabid beetles derive benefit from ruderal grasslands characterized by a higher nitrogen content and more complex canopies, as suggested by Venn et al. (2013).
Conservation efforts should focus primarily on the patches of semi-natural and ruderal habitats, which our study has shown to be suitable for important insect functional groups. Since the α-diversity indices are similar in both habitat types, there is a nested subset pattern, the insect functional groups are differently represented in the two habitats, and the effects of crucial factors on diversity act in different directions between the habitats, we can conclude that semi-natural and ruderal grasslands situated near one another play an important role in enhancing insect functional diversity in cities and post-industrial areas. Moreover, these habitats are characterized to some extent by specific α- and β-diversity patterns, which are more dissimilar than they would appear to be from their spatial proximity. Finally, we have demonstrated that this abandoned quarry maintains insect functional diversity in the city centre since it provides a mosaic of spontaneously established semi-natural and ruderal grasslands. As insect assemblages contain umbrella and keystone species such as ants and butterflies, their conservation improves biodiversity conservation in urban landscapes (Nowicki et al. 2007). Areas like abandoned quarries can enrich biodiversity not only in urban areas, but also on a much larger geographical spatial scale (γ-diversity) (Sattler et al. 2011), as they support different types of vegetation offering specific environmental conditions. However, since our study was based on a single abandoned quarry, comparative studies at larger spatial scales are required. Land managers should consider whether the creation of parks and artificial greenery on post-industrial sites is beneficial for biodiversity conservation and cost-effective, since a mosaic of semi-natural and ruderal habitats already occurs there (cf. Goddard et al. 2010). Basic conservation actions should involve the preservation of habitat mosaics of semi-natural and ruderal sites in urban areas, and the rotational mowing of both habitat types (Cizek et al. 2012), although mowing should be less frequent on ruderal sites, i.e. one habitat patch should be mown once every 3 years.
References
Akeboshi A, Takagi S, Murakami M, Hasegawa M, Miyashita T (2015) A forest–grassland boundary enhances patch quality for a grassland-dwelling butterfly as revealed by dispersal processes. J Insect Conserv 19(1):15–24
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Angold PG, Sadler JP, Hill MO, Pullin A, Rushton S, Austin K, Small E, Wood B, Wadsworth R, Sanderson R, Thompson K (2006) Biodiversity in urban habitat patches. Sci Total Environ 360:196–204
Antrop M, Van Eetvelde V (2000) Holistic aspects of suburban landscapes: visual image interpretation and landscape metrics. Landsc Urban Plan 50:43–58
Atmar W, Patterson BD (1993) The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 96:373–382
Atmar W, Patterson BD (1995) The nestedness temperature calculator: a visual basic program including 294 presence–absence matrices. AICS Research, University Park
Aviron S, Nitsch H, Jeanneret P (2009) Ecological cross compliance promotes farmland biodiversity in Switzerland. Front Ecol Environ 7:247–252
Bétard F (2013) Patch-scale relationships between geodiversity and biodiversity in hard rock quarries: case study from a disused quartzite quarry in NW France. Geoheritage 5:59–71
Borer ET, Seabloom EW, Tilman D (2012) Plant diversity controls arthropod biomass and temporal stability. Ecol Lett 15:1457–1464
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. Springer, New York
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74(1):46–69
Cizek O, Zamecnik J, Tropek R, Kocarek P, Konvicka M (2012) Diversification of mowing regime increases arthropods diversity in species-poor cultural hay meadows. J Insect Conserv 16:215–226
Clarke KM, Fisher BL, Le Buhn G (2008) The influence of urban park characteristics on ant (Hymenoptera Formicidae) communities. Urban Ecosyst 11:317–334
Concepción ED, Diaz M, Baquero RA (2008) Effects of landscape complexity on the ecological effectiveness of agri-environment schemes. Landsc Ecol 23:135–148
Denys C, Schmidt H (1998) Insect communities on experimental mugwort (Artemisia vulgaris L) plots along an urban gradient. Oecologia 113:269–277
Driscoll DA (2008) The frequency of metapopulations, metacommunities and nestedness in a fragmented landscape. Oikos 117:297–309
Dubiel E (2005) Mapa zbiorowisk roślinnych III Kampusu Uniwersytetu Jagiellońskiego i okolic Instytut Botaniki Uniwersytetu Jagiellońskiego Kraków
Duelli P, Obrist MK, Schmatz DR (1999) Biodiversity evaluation in agricultural landscapes: above-ground insects. Agric Ecosyst Environ 74:33–64
Fahrig L (2003) The effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol S 4:487–515
Fischer J, Lindenmayer DB (2005) Nestedness in fragmented landscapes: a case study on birds arboreal marsupials and lizards. J Biogeogr 32:1737–1750
Galluzzi G, Eyzaguirre P, Negri V (2010) Home gardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodivers Conserv 19:3635–3654
Gilbert OL (1989) The ecology of urban habitats London. Chapman and Hall, New York
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25(2):90–98
Gonthier DJ, Ennis KK, Farinas S, Hsieh H, Iverson AL, Batáry P, Rudolphi J, Tscharntke T, Cardinale BJ, Perfecto I (2014) Biodiversity conservation in agriculture requires a multi-scale approach. Proc Biol Sci 281:20141358
Hammer Ř, Harper DAT, Ryan PD (2001) (PAST) Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1) 9pp http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hanski I (1999) Metapopulation ecology. Oxford University Press, New York
Harper DAT (ed) (1999) Numerical palaeobiology. Computer-Based Modelling and Analysis of Fossils and their Distributions. John Wiley and Sons, Chichester, New York, Weinheim, Brisbane, Singapore, Toronto
Helden AJ, Leather SR (2004) Biodiversity on urban roundabouts - Hemiptera management and the speciesarea relationship. Basic Appl Ecol 5(4):367–377
Helden AJ, Gemma C, Stamp CG, Leather RS (2012) Urban biodiversity: comparison of insect assemblages on native and non-native trees. Urban Ecosyst 15:611–624
Hunter MR, Hunter MD (2008) Designing for conservation of insects in the built environment. Insect Conserv Divers 1(4):189–196
Hurvich CM, Tsai C (1989) Regression and time series model selection in small samples. Biometrika 76:297–307
Kalarus K, Nowicki P (2015) How do landscape structure management and habitat quality drive the colonization of habitat patches by the dryad butterfly (Lepidoptera: Satyrinae) in fragmented grassland? PLoS ONE 10(9):e0138557
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. Nairobi World Agroforestry Centre (ICRAF)
Kruess A, Tscharntke T (1994) Habitat fragmentation species loss and biological control. Science 264:1581–1584
Kudłek J, Pępkowska A, Walasz K, Weiner J (2005) Koncepcja ochrony różnorodności biotycznej Krakowa Uniwersytet Jagielloński Kraków
Lee KN (2007) An urbanizing world in: state of the world 2007: our urban future. World Watch Institute, Washington DC, pp 3–21
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, New York
Lizée HM, Manel S, Mauffrey JF, Thierry Tatoni T, Deschamps-Cottin M (2012) Matrix configuration and patch isolation influences override the species–area relationship for urban butterfly communities. Landsc Ecol 27:159–169
Luck GW, Smallbone LT (2010) Species diversity and urbanisation: patterns drivers and implications. In: Gaston KJ (ed) Urban ecology. Cambridge University Press, Cambridge, pp 88–119
Maes D, Van Dyck H (2005) Habitat quality and biodiversity indicator performances of a threatened butterfly versus a multispecies group for wet heathlands in Belgium. Biol Conserv 123:177–187
Manning PM, Slade ME, Beynon AS, Lewis TO (2016) Functionally rich dung beetle assemblages are required to provide multiple ecosystem services. Agric Ecosyst Environ 218(15):87–94
Matteson KC, Grace JB, Minor ES (2013) Direct and indirect effects of land use on floral resources and flower-visiting insects across an urban landscape. Oikos 122:682–694
McIntyre EN (2000) Ecology of urban arthropods: a review and a call to action ecology and population biology. Ann Entomol Soc Am 93(4):825–835
Moroń D, Lenda M, Skórka P, Szentgyörgyi H, Settele J, Woyciechowski M (2009) Wild pollinator communities are negatively affected by invasion of alien goldenrods in grassland landscapes. Biol Conserv 142:1322–1332
Nakamura A, Catterall CP, House APN, Kitching RL, Burwell CJ (2007) The use of ants and other soil and litter arthropods as bio-indicators of the impacts of rainforest clearing and subsequent land use. J Insect Conserv 11:177–186
Natuhara Y, Imai C, Takahashi M (1999) Pattern of land mosaic affecting butterfly assemblage at mt Ikoma, Osaka, Japan. Ecol Res 14:105–118
Niemelä J, Kotze DJ (2009) Carabid beetle assemblages along urban to rural gradients: a review. Landsc Urban Plan 92:65–71
Niemelä J, Kotze DJ, Venn S, Penev L, Stoyanov I, Spence J, Hartley D, de Oca EM (2002) Carabid beetle assemblages (Coleoptera Carabidae) across urban-rural gradients: an international comparison. Landsc Ecol 17:387–401
Nowicki P, Pepkowska A, Kudlek J, Skórka P, Witek M, Settele J, Woyciechowski M (2007) From metapopulation theory to conservation recommendations: lessons from spatial occurrence and abundance patterns of Maculinea butterflies. Biol Conserv 140:119–129
Obrist MK, Duelli P (2010) Rapid biodiversity assessment of arthropods for monitoring average local species richness and related ecosystem services. Biodivers Conserv 19:2201–2220
Oliver I, Beattie AJ (1995) Invertebrate morphospecies as surrogates for species: a case study. Conserv Biol 10:99–109
Pulliam HR, Danielson BJ (1991) Sources sinks and habitat selection: a landscape perspective on population dynamics. Am Nat 137:50–66
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Core Team (2017) R: A language and environment for statistical computing R Foundation for Statistical Computing Vienna Austria URL https://www.R-project.org/
Rader et al (2016) Non-bee insects are important contributors to global crop pollination. PNAS 113:146–151
Rangel TFLVB, Diniz-Filho AF, Bini LM (2010) SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33:46–50
Řehounková K, Čížek L, Řehounek J, Šebelíková L, Tropek R, Lencová K, Bogusch P, Marhoul P, Máca J (2016) Additional disturbances as a beneficial tool for restoration of post-mining sites: a multi-taxa approach. Environ Sci Pollut Res 23:13745–13753
Ricketts T, Imhoff M (2003) Biodiversity urban areas and agriculture: locating priority ecoregions for conservation. Conserv Ecol 8(2):1
Roy AH, Rosemond AD, Paul MJ, Leigh DS, Wallace JB (2003) Stream macroinvertebrate response to catchment urbanisation (Georgia USA). Freshw Biol 48:329–346
Sattler T, Duelli P, Obrist MK, Arlettaz R, Moretti M (2010) Response of arthropod species richness and functional groups to urban habitat structure and management. Landsc Ecol 25:941–954
Sattler T, Obrist KM, Duelli P, Moretti M (2011) Urban arthropod communities: added value or just a blend of surrounding biodiversity? Landsc Urban Plan 103(3–4):347–361
Schouten MA, Verweij PA, Barendregt A, Kleukers RJM, De Ruiter PC (2007) Nested assemblages of Orthoptera species in the Netherlands: the importance of habitat features and life-history traits. J Biogeogr 34:1938–1946. https://doi.org/10.1111/j.1365-2699.2007.01742.x
Skórka P, Settele J, Woyciechowski M (2007) Effects of management cessation on grassland butterflies in southern Poland. Agric Ecosyst Environ 121:319–324
Skórka P, Lenda M, Moroń D, Kalarus K, Tryjanowski P (2013) Factors affecting road mortality and the suitability of road verges for butterflies. Biol Conserv 159:148–157
Snep RPH, Opdam PFM, Baveco JM, WallisDeVries MF, Timmermans W, Kwak RGM, Kuypers V (2006) How peri-urban areas can strengthen animal populations within cities: a modeling approach. Biol Conserv 127:345–355
Thompson B, McLachlan S (2007) The effects of urbanization on ant communities and myrmecochory in Manitoba, Canada. Urban Ecosyst 10:43–52
Tilman D, Reich PB, Knops JMH (2006) Biodiversity and ecosystem stability in a decadelong grassland experiment. Nature 441:629–632
Tropek R, Kadlec T, Karesova P, Spitzer L, Kocarek P, Malenovsky I, Banar P, Tuf IH, Hejda M, Konvicka M (2010) Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants. J Appl Ecol 47:139–147
Tscharntke T, Bommarco R, Clough Y, Crist TO, Kleijn D, Rand TA, Tylianakis JM, Nouhuys V, Vidal S (2007) Conservation biological control and enemy diversity on a landscape scale. Biol Control 43:294–309
Venn SJ, Kotze DJ, Lassila T, Niemelä JK (2013) Urban dry meadows provide valuable habitat for granivorous and xerophilic carabid beetles. J Insect Conserv 17:747–764
Vonshak M, Dayan T, Kronfeld-Schor N (2009) Arthropods as a prey resource: patterns of diel seasonal and spatial availability. J Arid Environ 73:458–462
WallisDeVries MF, Poschlod P, Willems JH (2002) Challenges for the conservation of calcareous grasslands in northwestern Europe: integrating the requirements of flora and fauna. Biodivers Conserv 104:265–273
Witmer JE, Hough-Goldstein JA, Pesek JD (2003) Grounddwelling and foliar arthropods in four cropping systems. Environ Entomol 32:366–376
Yan Chong K, Teo S, Kurukulasuriya B, Fei Chung Y, Rajathurai S, Tiang Wah Tan H (2014) Not all green is as good: different effects of the natural and cultivated components of urban vegetation on bird and butterfly diversity. Biol Conserv 171:299–309
Acknowledgements
The authors are grateful to Magdalena Droździewicz for her assistance with the coordination of the fieldwork. We would also like to express our warm appreciation to the students from KPSt UJ (Student Naturalist Society at the Jagiellonian University) for their participation in the fieldwork and to Peter Senn for improving the English of the manuscript. On behalf of all the authors, the corresponding author states that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kalarus, K., Halecki, W. & Skalski, T. Both semi-natural and ruderal habitats matter for supporting insect functional diversity in an abandoned quarry in the city of Kraków (S Poland). Urban Ecosyst 22, 943–953 (2019). https://doi.org/10.1007/s11252-019-00869-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-019-00869-3