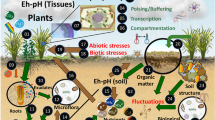
Abstract
Background
Oxidation-reduction and acid–base reactions are essential for the maintenance of all living organisms. However, redox potential (Eh) has received little attention in agronomy, unlike pH, which is regarded as a master variable. Agronomists are probably depriving themselves of a key factor in crop and soil science which could be a useful integrative tool.
Scope
This paper reviews the existing literature on Eh in various disciplines connected to agronomy, whether associated or not with pH, and then integrates this knowledge within a composite framework.
Conclusions
This transdisciplinary review offers evidence that Eh and pH are respectively and jointly major drivers of soil/plant/microorganism systems. Information on the roles of Eh and pH in plant and microorganism physiology and in soil genesis converges to form an operational framework for further studies of soil/plant/microorganism functioning. This framework is based on the hypothesis that plants physiologically function within a specific internal Eh-pH range and that, along with microorganisms, they alter Eh and pH in the rhizosphere to ensure homeostasis at the cell level. This new perspective could help in bridging several disciplines related to agronomy, and across micro and macro-scales. It should help to improve cropping systems design and management, in conventional, organic, and conservation agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“What drives life is a little electric current, kept up by the sunshine” was the elegant summary of Albert Szent-Gyorgyi (1960), a Nobel prize laureate in physiology. Indeed, electrons are the essential reactants in inorganic, organic, and biochemical reactions (Bohn 1971). The chemistry of living organisms relies even more on oxidation-reduction reactions, i.e., transfer of electrons, than it does on acid–base reactions, which are more focused on proton transfers (Clark 1960; Dietz 2003; Falkowski et al. 2008; Greenberg 1998). It is worth reviewing that the main constituents of living organisms, especially proteins, are just six elements: (i) oxygen; the strongest oxidizing agent; (ii) hydrogen; the strongest reducing agent; and (iii) the four elements that have the largest amplitude in redox numbers: carbon (−IV in CH4 to + IV in CO2), nitrogen (−III in NH +4 to + V in NO -3 ), phosphorus (−III in PH3 to + V in PO 3-4 ), and sulfur (−II in H2S to + VI in SO 2-4 ).
Oxidation-reduction conditions are classically assessed by measuring the redox potential (Eh), expressed in volts. The zero point for the Eh scale is set by the standard hydrogen electrode (SHE), involving the redox couple H+/H2. Eh is commonly used in a large panel of disciplines dealing with living organisms, such as microbial ecology (Alexander 1964), geochemistry, biogeochemistry, limnology (Bohn 1971; Falkowski et al. 2008; Reddy and DeLaune 2008), bioenergetics (Guérin 2004; Mathis 1995; Szent-Gyorgyi 1957), hydrobiology and the study of marine ecosystems (Meadows et al. 1994), soil science (Chadwick and Chorover 2001), and physiology and ecophysiology (De Gara et al. 2010; Dessaux et al. 2009; Dietz 2003; Foyer and Noctor 2005; Lambers et al. 2008). According to the discipline, Eh and pH get measured on different scales and for various substrata: organelles, cells, plants, rhizosphere, bulk soil, sediments, soil solution, or water.
However, in many disciplines, oxidation-reduction conditions and electron fluxes have not received the same attention as have pH and proton fluxes. In soil science, for instance, despite the recognition of the importance of Eh, pH is often regarded as the master variable (Brady and Weil 2010; Simek and Cooper 2002). This is also the case in plant physiology (Rengel 2002). Surprisingly, and in contrast to pH, Eh is much less central in agronomy. Most studies on soil Eh have remained limited to extreme situations leading to anaerobiosis, as in paddy fields and submerged soils (Bartlett and James 1993; De Mars and Wassen 1999; Gotoh and Yamashita 1966; Kludze and DeLaune 1999; Kogel-Knabner et al. 2010; Ponnamperuma 1965; 1972; Stepniewski and Stepniewska 2009; Yoshida 1981).
The Eh of aerobic soils, which are the majority of cultivated soils, has received little attention in agronomy. Three methodological reasons might explain this lack of references in the literature: (i) the high variability of Eh in space and time, especially when compared to pH variability; (ii) the difficulties in measuring Eh in aerobic soils; and (iii) the dependence of Eh and pH. Consequently, Eh measurements are difficult to reproduce and interpret, and results from various authors are difficult to compare (Snakin et al. 2001).
Despite the paucity of redox potential (Eh) studies in agronomy, especially for aerobic soils, this essential parameter should not be overlooked because it is difficult to measure or interpret. It is quite possible that the lack of studies of Eh on aerobic soils may account for an a priori underestimation of the impact that high levels of Eh can have on soil//plant/microorganism functioning and on plant health and production. Agronomists are quite possibly missing a key factor in plant and soil science which, when associated systematically with pH, could help advance agronomic knowledge for sustainable agriculture.
This paper undertakes: (i) to review the existing literature on redox potential (Eh) in various disciplines connected to agronomy, associated or not with pH; and (ii) to integrate this knowledge to construct an operational perspective for bridging disciplines related to agronomy and for linking micro and macro-scales, from plant cells to plants, roots and soil on a field scale.
The paper proceeds through five sections. First, I review and underscore the importance of Eh at cell and plant levels in physiology. In the second section, the respective and combined influence of Eh and pH on microorganisms are explored. In the third section, I successively explore at field scale: (i) Eh and pH ranges and variability; (ii) the influence of Eh and pH on plant growth; (iii) the relationship between soil Eh-pH and nutrient availability for plants; (iv) the impact of plants on soil Eh and pH; (v) the interactions between Eh-pH and soil organic matter; (vi) the relationship between Eh-pH and soil genesis; and (vii) the influence of Eh and pH on greenhouse gas emissions, soil pollution, and bioremediation. The fourth section shows the impact of agricultural practices on soil Eh and pH. The fifth section synthesizes this knowledge, raising the hypothesis that plants, along with microorganisms, modify the Eh and pH in the rhizosphere to adjust for an optimum physiological level at which they can function well. The questions this composite perspective raises for agronomy are then discussed. I suggest that this perspective could help in assessing agricultural practices, providing a conceptual framework for developing tools for cropping systems design and management, in conventional, organic and conservation agriculture.
Oxidation and reduction in cell and plant physiology
Oxidation-reduction reactions and energy
In the plant kingdom, photosynthesis uses the light energy of photons to combine (reduce) carbon dioxide from the air with hydrogen taken from water (Govindjee and Krogmann 2004; Wurmser 1921). This reaction produces glucose and releases oxygen:
It is an endothermic reaction, with a variation in enthalpy ΔH = + 470 kJ per mol of CO2 at 25 °C (Bisio and Bisio 1998).
Nitrogen fixation also is an endothermic reduction of atmospheric nitrogen (Eagleson 1993), using energy captured by photosynthesis in glucose which behaves as an energy reservoir (Atkins and Jones 1997). The overall equation for the reduction of nitrogen by glucose (Bayliss 1956) is:
Similarly, proteosynthesis and liposynthesis in plants are reduction reactions, with an accumulation of chemical energy in the synthesized molecules. The energy released by the oxidation of these varied reduced compounds in an oxygenated atmosphere is used by cells (Lambers et al. 2008). The energy functioning of cells relies on the Krebs cycle in the mitochondria: substrates imported into the mitochondrial matrix are oxidized in a cyclical process, generating reducing power (Lambers et al. 2008).
When these oxidation-reduction reactions are catalyzed, this modifies their kinetics, but not their thermodynamic conditions. Among the thermodynamically possible reactions, those which predominate are determined by their redox kinetics. The thermodynamics and kinetics of oxidation-reduction reactions are therefore essential for understanding the biochemical functioning of cells and living organisms.
Compartmentalization and redox state
The myriad of inter-actions between redox-active compounds, and the effect of environmental parameters on them, has been encapsulated in the concept of ‘cellular redox state’. Cellular redox state is envisaged as the sum of reducing and oxidizing redox-active molecules (Potters et al. 2010). Derived from this concept of a cellular redox state are the organellar redox states as mitochondria redox state, the tissue and even the organ redox states (Potters et al. 2010).
Within a cell, the various organelles operate properly at a different redox state. For instance, maintenance of a reduced nuclear redox state is critical for transcription factor binding in transcriptional activation (Hansen et al. 2006). Ordered by their redox status, in a cell with no functional chloroplasts or a photosynthetic cell in the dark, mitochondria operate at the lowest Eh level, followed by, respectively, nuclei, cytosol, endoplasmic reticulum, and extracellular space (Hansen et al. 2006).
The redox situation is further complicated in plants by their highly reactive photosynthetic metabolism (Dietz 2003). In plants, photosynthesis generates redox intermediates with extraordinarily negative redox potentials. Light-driven electron transport transfers electrons from the acceptor site of photosystem I (mid-point potential < −900 mV) to various acceptors, including oxygen (Baier and Dietz 2005; Blankenship 2002).
Furthermore, photosynthesis in the chloroplasts involves membrane-bound photosynthetic electron transport, implying differences in Eh and pH: (i) between the two sides of the membrane as the lumen and the stroma for the thylakoids; but also (ii) between different areas of the membrane surface (Lambers et al. 2008). Similar processes are found in the Krebs cycle in the mitochondria. Thus, compartmentalization and proper poising of the redox potential are critical not only in establishing transfer kinetics in many instances, but also in conserving the energy inherent in the potential gradients associated with electron transport pathways (Chang and Swenson 1997).
As a consequence, the redox state is a critical determinant of cell functioning, and any major imbalances can cause severe damage or death (Dietz and Scheibe 2004). In plants such as Nicotiana sylvestris, leaf mitochondria modulate the whole cell redox homeostasis and determine antioxidant capacity (Dutilleul et al. 2003).
Peroxidation is particularly harmful to cells. For example, peroxidation leads to disruption of membranes, oxidation of thiol groups, inhibition of thiol-containing enzymes, and DNA strand breaks (Ahmad 1992). Oxidation of mitochondrial glutathione jeopardizes mitochondrial integrity, causes oxidation of pyridine nucleotides, and ultimately impairs energy production. Peroxidation of membrane lipids causes cell dysfunction and ultimately cell death (Ahmad 1992).
The production of reactive oxygen and reactive nitrogen species in plant cells can lead to an alteration of proteins through the oxidation of amino acid side groups (Foyer and Noctor 2005). Peroxidation also inhibits a large variety of enzymes as they are regulated by the oxidation-reduction state (Ahmad 1992; Chang and Swenson 1997; Ding et al. 1996; Ghezzi 2005). Severe protein oxidation is costly to the cell since oxidatively-damaged proteins need to be degraded by specific proteases (Sweetlove et al. 2009). Conversely, dissipatory pathways are required to avoid any detrimental effects caused by over-reduction of the cellular redox state (Scheibe et al. 2005).
Redox homeostasis
Plants perform photosynthesis and assimilatory processes in a continuously changing environment. Rapidly fluctuating environmental conditions can significantly stress organisms, particularly when fluctuations cross the thresholds of normal physiological tolerance (DeAngelis et al. 2010). To prevent these huge fluxes from causing catastrophic oxidative damage, there is an extensive network of compensatory, buffering mechanisms (Dietz 2003; Hanke et al. 2009; Hansen et al. 2006; Kandlbinder et al. 2003; Lambers et al. 2008; Noctor et al. 2000).
The simultaneous presence of strong oxidants and strong reductants during oxygenic photosynthesis is the basis for regulation (Scheibe et al. 2005). Glutathione, glutaredoxins and ascorbate are involved in a large variety of cellular processes and play a crucial role in response to oxidative stress and control of reactive oxygen species (ROS) (Foyer and Noctor 2003; Mullineaux and Rausch 2005; Noctor and Foyer 1998; Sanchez-Fernandez et al. 1997; Xing et al. 2006). Furthermore, proteins may have an over-representation of easily oxidizable amino acids (as cysteine, tyrosine, and tryptophan) on their surface to act as a decoy or as sacrificial residues, thus preventing or postponing oxidation of residues that are more important for the function of the protein (Saurina et al. 2000; Sweetlove et al. 2009).
These buffering mechanisms must be integrated with signaling cascades in a greater redox network, to ensure that short-term responses are adequate and that, if buffering capacity is exceeded, there is a response at transcript level (Hanke et al. 2009). Short-term and long-term mechanisms interact with each other in a flexible way, depending on intensity and the type of impact (Scheibe et al. 2005). Cellular homeostasis will be maintained as long as the mechanisms for acclimation are present in sufficiently high capacities. If an impact is too rapid, and acclimation at the level of gene expression cannot occur, cellular damage and cell death are initiated (Scheibe et al. 2005).
In nodules harboring symbiotic rhizobia, leguminous plants provide bacteria with energy and a micro-aerobic environment compatible with nitrogenase activity (Marino et al. 2009). Oxygen can be either beneficial or detrimental for diazotrophy in organisms capable of an aerobic catabolism. It supports the production of a substrate for nitrogenase (ATP), but it can also inhibit the activity and repress the synthesis of this enzyme (Hill 1988). ROS are produced at every step of the symbiotic association: during symbiosis establishment, during nitrogen fixation, and during nodule senescence. In order to deal with this ROS production, nodules are fitted with a large panel of enzymatic and non-enzymatic antioxidant mechanisms (Marino et al. 2009).
Redox regulation, redox signaling, plant phenology, and global environment sensing
Redox compartmentalization also functions as a mechanism for specificity in redox signaling (Hansen et al. 2006). Increasing evidence shows the importance of redox regulation and signaling in the context of transport activities, plant development, and programmed cell death; it also indicates involvement of redox interactions in proton pumping, membrane energization, ion channel regulation, iron reduction, nutrient uptake, signal transduction, and growth regulation (Dietz 2003; Dietz and Pfannschmidt 2011; Kandlbinder et al. 2004; Luthje et al. 1997; Noctor 2006; Pfannschmidt 2003).
Redox signals also regulate protein-DNA interactions and play a key role in gene expression, DNA replication, and genome stability (Shlomai 2010; Turpaev and Litvinov 2004). Photosynthesis is also governed by redox on essentially all levels, ranging from gene transcription to translation, assembly and turnover, as well as short-term adaptation by state transition and enzyme activity (Dietz 2003).
The redox regulation of many enzymes has a marked incidence on plant development. As early as 1949, Stout observed that induction of the reproductive development of sugar beet is correlated with changes in the oxidation-reduction balance of certain regions of the plant (Stout 1949). Root growth, flowering, development of floral organs, leaf sectoring, and photoperiodism are regulated by the redox potential (Becker et al. 2006; Foreman et al. 2003; Rosso et al. 2009; Sanchez-Fernandez et al. 1997; Xing et al. 2006). Reactive oxygen species (ROS) are key actors in the regulation of plants' dormancy and germination (Bailly et al. 2008). Cellular antioxidants influence plant growth and development by modulating processes from mitosis and cell elongation to senescence and death (Foyer and Noctor 2005).
It is now well established that redox regulation is a central element in adjusting plant metabolism and development to the prevailing environmental conditions (Dietz 2003). Redox sensing/signaling mechanisms may be primary sensors of environmental change and are an important component for sensing abiotic stresses in general (Huner et al. 1996). For instance, cellular redox status, associated with cold-stress sensing, can activate redox-responsive proteins and might act as a signal for the reconfiguration of gene expression (Yadav 2010). Chloroplastic redox-sensing affects chloroplastic and nuclear gene expression in response, not only to light intensity, but to a myriad of abiotic stresses (Baier and Dietz 2005; Wilson et al. 2006).
Finally, the nutritional status of a plant influences its redox status. N-, P- or S-nutrient deprivation triggers distinct redox changes and induces oxidative stress with a rather defined pattern in the context of nutrient-specific alterations in metabolism. For instance, N-deprivation caused a five-fold increase in ascorbic acid in Arabidopsis thaliana leaves (Kandlbinder et al. 2004). P-starvation induced an increase in ascorbate and glutathione levels. Sulfur depletion has been found to cause a drop in glutathione levels to less than 25 % of the control (Kandlbinder et al. 2004).
Redox signals, plant responses to biotic stresses, and resistance to pathogens
Redox activity plays a role in the biocontrol of plant pathogens (Altomare et al. 1999). Reactive oxygen species (ROS) in plants are known to accumulate during biotic stress, and different cellular compartments respond to them by a distinct antioxidant repertoire (Kuzniak 2010). A very widely-found local defense mechanism is the generation of ROS as a hypersensitive response of plants to pathogens and subsequent stress on the colonizing microbes or neighboring roots (Blokhina et al. 2003; Bolwell et al. 1995; Hartmann et al. 2009; Lamb and Dixon 1997; Mori and Schroeder 2004). Increased disease resistance is very likely to be a combinatorial effect of redox signals triggered by salicylic acid, H2O2, glutathione, and potentially additional yet unidentified compounds (Mateo et al. 2006). The ascorbate-glutathione (AsA-GSH) cycle serves as the main antioxidant pathway in plant cells, linking protection against ROS to redox-regulated plant defenses (Kuzniak 2010). On this basis, hydrogen peroxide could be regarded as an 'offensive weapon' of cells, and catalase as a 'defensive weapon,' as this enzyme degrades hydrogen peroxide into water and oxygen (Voisin 1959).
Oxidation-reduction processes are also involved in interactions between herbivorous insects and plants. Plants produce pro-oxidant compounds as an allelochemical defense, exacerbating the endogenous oxidative stress of all aerobic organisms (Ahmad 1992). For instance, host-plant phenolics have a widely recognized detrimental impact on gypsy moth (Lymantria dispar) (Meyer and Montgomery 1987; Rossiter et al. 1988; Roth et al. 1994). It has been suggested that oxidation of phenolics in the insect midgut produces toxic quinones, reducing food digestibility (Appel and Maines 1995). Herbivorous insects cope with this stress through direct detoxification of the prooxidants or antioxidant compounds and antioxidant enzymes (Ahmad 1992; Appel 1993; Roth et al. 1997). However, high levels of allelochemicals in plant foliage may overload the detoxification capacity of insects (Lindroth and Hemming 1990).
Redox potential, allelopathy, and recognition by parasitic plants
Among the 20,000 allelopathic substances identified, many are pro-oxidant and exert oxidative stress (Ahmad 1992; Downum 1986; Downum and Rodriguez 1986). For instance, the phytotoxic action of Tagetes minuta is primarily the result of increased lipid peroxidation rates, with the essential oils acting as an ‘oxidant’ agent (Scrivanti et al. 2003).
Similarities exist between allelopathic phenomena and those of plant recognition by parasitic plants (Tomilov et al. 2006). Parasite perception of host factors by parasitic plants of the Scrophulariaceae family such as Striga sp. occurs via a redox-associated mechanism (Yoder 2001).
Redox potential and pH in plant cell and physiology: the ignored Eh-pH interaction
Cell pH, regarded as the master variable in plant physiology, has been extensively studied and reviewed (Kurkdjian and Guern 1989; Rengel 2002; Smith and Raven 1979). Hence, it is not reviewed in this paper. Like Eh and electron transfers, pH and proton transfers are crucial in the energy functioning of cells and strongly affect plant metabolism and catabolism, as for example, embryogenic cell division (Pasternak et al. 2002). Water, as an active constituent in cell biology, is structured in a network which facilitates electron transfers between proteins and other biomolecules, and allows rapid proton diffusion (Ball 2008). As with Eh, compartmentalization and intracellular pH regulation are key features of cell physiology. Reactions in the cytosol are exquisitely sensitive to changes in pH (Taiz 1992). pH regulation involves very complex processes in interaction with each other (Felle 1988; Rengel 2002; Sakano 1998; Smith and Raven 1979). Cytosolic pH can be regulated by pumping massive amounts of protons out of the cytosol into the lumen of the vacuole, in which pH varies according to plant species (Smith and Raven 1979; Taiz 1992). Among the processes of pH regulation, Smith and Raven (1979) emphasize excretion of excess protons or hydroxyl ions to the root medium. As with Eh, pH-dependent signals regulate physiological processes as, for instance, root water transport during anoxic stress (Tournaire-Roux et al. 2003).
Surprisingly though, studies of Eh are generally disconnected from studies of pH; and Eh-pH interactions are ignored most of the time. This is clearly a limitation of most studies as Eh and pH are not independent influences; for instance, (i) oxidation-reduction reactions can involve a transfer of protons, especially the major chemical reactions involving changes in the oxidation state of Fe, Mn and N, which also imply the consumption or production of H+, and thereby a coupling of the Eh and pH (Hinsinger et al. 2003); (ii) a redox system on the plasma membrane of plant cells has implications for cytoplasmic pH regulation (Rengel 2002); and (iii) in the rhizosphere, redox processes are intimately coupled with pH changes and thus need to be taken into account for understanding all of the mechanisms underlying root-induced pH changes (Hinsinger et al. 2003). As a consequence, Eh and pH are best analyzed concomitantly, in interaction.
Eh, pH, and microorganisms
The Eh and pH of a milieu largely determine the metabolic types emergent in the bacterial community of that milieu, and they are therefore significant parameters for biological activity (Billen 1973; Stumm 1966). For instance, nitrogen fixation by Azospirillum spp. is governed by soil redox fluctuations, pH and organic matter (Charyulu and Rao 1980).
Eh clearly influences the development of microorganisms. As early as 1934, Heintze proposed using variations in soil Eh to characterize groups of microorganisms (Heintze 1934). Bacterial growth is directly correlated to changes in Eh (Kimbrough et al. 2006). Microbial and enzymatic activity are negatively correlated with Eh in anaerobic soils (Brzezinska 2004; Kralova et al. 1992; Snakin and Dubinin 1980). Furthermore, the redox state of nodules is regarded as a referee of legume-rhizobium symbiosis (Marino et al. 2009).
Each microorganism type is adapted to specific Eh conditions, and is characterized by its ability to develop within a wider or a narrower Eh-range. For instance, anaerobic bacteria can only develop within a narrow range of very low Eh values. Aerobic microorganisms such as Actinomyces sp. or Azotobacter sp. require a higher Eh, but can develop over a much wider range (Rabotnova and Schwartz 1962). Fungi develop more than bacteria under moderately reducing conditions (Eh > + 250 mV), while bacteria are more abundant than fungi under highly reducing conditions (Eh < 0 mV) (Seo and DeLaune 2010).
Frequent high-amplitude redox fluctuation may be a strong selective force on the phylogenetic and physiological composition of soil bacterial communities and may promote metabolic plasticity or redox-tolerance mechanisms (Pett-Ridge and Firestone 2005). For instance, indigenous soil bacteria are highly adapted to fluctuating redox regimens (Pett-Ridge and Firestone 2005). Microbial community acclimation or avoidance strategies for survival will, in turn, shape microbial community diversity and biogeochemistry (DeAngelis et al. 2010).
Although studies on the impact of Eh on plant pathogens are few, they all indicate that the development of several plant pathogens could be related to a high Eh. Four examples illustrate this hypothesis. First, the application of hydrogen peroxide to tobacco plants, or inhibition of catalase by the application of hydroxylamine (also leading to hydrogen peroxide accumulation in the cells), both induce development of the mosaic virus (Yamafuji and Cho 1948; Yamafuji and Fujiki 1947). Second, sclerotial differentiation in phytopathogenic fungi such as Rhizoctonia solani is induced by oxidative stress (Patsoukis and Georgiou 2007a, c). Third, it is known that soil-borne pathogens such as Fusarium sp. and Rhizoctonia solani can be controlled by a low Eh (Blok et al. 2000; Shinmura 2004; Takehara et al. 2004). Fourth, nitrogen fertilizer applied as NO -3 (oxidized form of N) triggers severe attacks of rice blast, whereas application as NH +4 (reduced form of N) does not induce rice blast (Osuna-Canizalez et al. 1991).
Similarly, each microorganism is adapted to a definite pH range outside of which it cannot live. Most soil bacteria develop between pH 4.5 and 10 (Baas Becking et al. 1960). Diversity is highest in neutral soils and minimum in acidic soils (Hinsinger et al. 2009; Lauber et al. 2009) The ecological behavior of microbes is determined, among other ways, by their ability to adapt themselves to wider or narrower pH ranges and by the limits of the pH range within which multiplication is possible (Rabotnova and Schwartz 1962). For instance, thio-bacteria have a very wide potential environment, while algae are found literally everywhere (Baas Becking et al. 1960). Saprophytic bacteria, which are widespread in soil and water, can exist under very diverse conditions (Rabotnova and Schwartz 1962). Highly ubiquitous Actinomycetes increase in alkaline soils (Roger and Garcia 2001), while Trichoderma prefer acid conditions (Steyaert et al. 2010). Thus, pH has been proposed as the main factor affecting the diversity and richness of soil microbial communities (Fierer and Jackson 2006; Lauber et al. 2009).
Microorganisms that are adapted to special locations and special ecological conditions, somewhat like pathogenic bacteria, mostly have a narrow pH range (Rabotnova and Schwartz 1962). Many pathogenic fungi develop preferably at a slightly low pH. For instance, development of Aphanomyces cochlioides et Pythium spp. was slowed down by a high pH (Payne et al. 1994). Fusarium wilt of flax was suppressed when the soil pH was raised above seven and clay was added (Höper et al. 1995), and Fusarium of Gerbera (Compositae) decreased when the pH was higher than six (Minuto et al. 2008).
Conversely, several studies have shown that virus development is slowed down by a low pH. In water, viruses are almost completely removed below a critical pH (Guan et al. 2003). In sediments, a strong correlation between virus abundance and pH indicates virus susceptibility to a low pH (Kyle et al. 2008). However, some viruses have been associated with Archaea, organisms living in extreme conditions as acidic hot springs (Happonen et al. 2010).
In plants, the activity of tomato spotted wilt and tobacco mosaic viruses, for instance, varied with pH (Best and Samuel 1936). Various explanations of the influence of low pH on virus development have been proposed, such as: (i) a glycoprotein, playing a crucial role in the capacity of the tomato spotted wilt virus to bind to sensitive cells, undergoes pH-dependent conformational changes at a low pH (Pekosz and Gonzalez-Scarano 1996; Whitfield et al. 2005); (ii) pH modifies the conductivity of the ion channel as has been observed in southern cowpea mosaic virus (Helrich et al. 2001); and (iii) pH induces RNA disaggregation and degradation in the case of turnip yellow mosaic virus (Sam et al. 1991).
At the same time, microorganisms are known to be able to modify the Eh and the pH of their surrounding medium, depending on their requirements; further, they have this ability to a considerably greater degree than other organisms (Rabotnova and Schwartz 1962). Potter (1911) was probably the first to draw attention to the fact that microbial cultivation generates a lowering of the Eh. In soils, widely-diffused saprophytes and pathogenic anaerobes lower the Eh (Rabotnova and Schwartz 1962). In aerobic soils, microorganisms consume oxygen and, as a consequence, lower the Eh (Bohrerova et al. 2004; Kralova et al. 1992). When soil moisture content increases Eh decreases, leading to anaerobic soil conditions because of the rapid consumption of oxygen by microbes and the resulting partial or total loss of oxygen (Savant and Ellis 1964).
In a culture medium, the pH value originally present is changed and is regulated by the metabolism of the microorganisms (Rabotnova and Schwartz 1962). Fungi can influence soil conditions such as the pH within the microenvironment surrounding their hyphae (Garrett 1963; Twining et al. 2004). Microorganism activity usually leads to acidification (Rabotnova and Schwartz 1962). In soils, this acidification can be compensated for by the buffering capacity of the absorbing complex (Roger and Garcia 2001).
Soil Eh and pH at the field scale
Soil Eh and pH variability
Soil Eh fluctuates normally between −300 and +900 mV. Waterlogged soils have an Eh below +350 to +250 mV and dry soils above +380 to +400 mV, according to various authors (Pearsall and Mortimer 1939; Pezeshki 2001). For Kaurichev and Shishova (1967), four main classes of soil conditions can be determined according to Eh: Aerated soils have an Eh over + 400 mV; moderately reduced soils between +100 and +400 mV; reduced soils between −100 and +100 mV; and highly reduced soils between −100 and −300 mV. Cultivated soils are most frequently in the range of +300 and +500 mV under aerobic conditions (Macías and Camps Arbestain 2010). However, Eh can reach +750 mV in podzols (Kaurichev and Shishova 1967).
Most cultivated soils have a pH between 4 and 9, but a pH below 3 and above 10 can be measured in acid sulfate soils or sodic soils, respectively (Brady and Weil 2010). Interestingly, pH and Eh are negatively correlated in soils (Bohrerova et al. 2004; Van Breemen 1987).
Soil Eh and pH can greatly vary at very short distances (Hinsinger et al. 2009; Yang et al. 2006). For instance, the center of a humid soil aggregate of 6–7 mm in diameter can have a redox potential 100 to 200 mV lower than its surface (Kaurichev and Tararina 1972). Various authors have also reported marked differences in Eh and pH as a function of soil depth (Bohrerova et al. 2004; Mansfeldt 2003; Rousseau 1959; Snakin et al. 2001). For instance, in a grey forest soil in Russia, in the A horizon at a depth of 7 cm, Eh and pH were respectively measured at 80 mV lower and 0.9 units higher than in the C horizon at a depth of 116 cm (Snakin et al. 2001). In addition, the living matter in soil creates significant Eh and pH heterogeneity within a given soil horizon (Snakin et al. 2001).
Soil Eh and pH also exhibit high temporal variability, with a daily cycle and strong seasonal influences (Mansfeldt 2003; Snakin et al. 2001). Sabiene et al. (2010) also documented inter-annual variations in relation to climatic conditions and soil moisture. Flooding dramatically affects both Eh and pH, especially in organic soils (Kashem and Singh 2001). Balakhnina et al. (2010) reported a very rapid decrease in soil Eh from + 543 to +70 mV within a few hours after flooding, and a restoration of the initial level within a few days after drainage.
Redox kinetics are largely governed by microbial catalysis as soil microorganisms produce catalytic enzymes (Fenchel et al. 1998). Microbial activity is itself influenced by Eh and pH. It is also promoted by clay, which plays a role of surface catalyst (Filip 1973; Theng and Orchard 1995), retains organic matter used by microorganisms (Wardle 1992), and contributes to the development of protective micro-habitats (Heijnen and van Veen 1991). In a sandy soil a significant increase of the microbial biomass (bacteria and fungi) was observed when it was enriched with clay (Davet 1996).
Faced with this high temporal variability, the capacity of a soil to buffer Eh and pH is regarded as an important parameter by various authors. Eh buffering capacity determines the evolution of the soil's oxidation-reduction conditions, and especially the speed and the amplitude of the response of a soil to an input of electrons (Von de Kammer et al. 2000). These previous authors proposed calculation of a parameter, total reducing capacity (TRC), as the number of electrons that can be provided by the reduced component of a milieu. Similarly, Heron et al. (1994) calculated an oxidation capacity (OXC) taking into account the oxygen, nitrate, iron, manganese and sulfates available as the main electron acceptors. This oxidation capacity corresponds to the quantity of electrons that can be accepted by a milieu.
Soil Eh-pH and plant growth
Eh has received little attention in the whole-plant context, apart from the influence of having very low redox potential in submerged soils. Eh limits for plant growth are between +300 and +700 mV (Volk 1993). Reduced conditions (< +350 mV) are particularly limiting for many plants. For instance, the richness of riparian plant species and total plant cover were positively correlated with Eh at a depth of 10 and 25 cm (Dwire et al. 2006). Five-year-old trees had greater vertical growth and higher survival rates in transition zones with Eh varying between +400 and +450 mV than in lowlands with Eh varying between +250 and +380 mV (Pennington and Walters 2006). Annual sugarcane yields decreased by 0.2 to 0.3 t/ha per day when Eh was lower than 332 mV (Carter 1980). In acid sulfate soils in Vietnam, rice yields increased sharply when Eh rose under reduced conditions, and conversely decreased when Eh rose under oxidized conditions: the highest rice yield was obtained in soils forming jarosite around rice roots, indicating local Eh values of approximately +400 mV (Husson et al. 2001; Husson et al. 2000b).
The tolerance of plants to changing redox conditions varies greatly. A study of riparian meadows suggests that the observed biological diversity is strongly related to steep environmental gradients in hydrology and soil Eh (Dwire et al. 2006). Similarly, close associations were observed between distinct plant community zones and Eh in created wetlands, suggesting that natural plant communities may be used to characterize the oxidation status of soils in a broad range of wetland ecosystems (Pennington and Walters 2006).
The pH of the soil solution is also a critical environmental factor for plant growth (Brady and Weil 2010). As for redox conditions, the tolerance of plants to acid and/or alkaline conditions varies considerably; however, they all have a rather narrow range of optimum pH conditions. Most cultivated crop plants grow well on soils that are just slightly acid to near-neutral, and only a few species can develop at a pH below 4.5 or above 9 (Brady and Weil 2010). Some plants such as sweet potato (Ipomea batatas), cassava (Manihot esculenta), and tea (Camellia sinensis) are known for their ability to grow under acid soil conditions. Strong acidity or highly alkaline conditions affect plant growth, mainly because pH strongly influences nutrient availability and the risk of ion toxicity (Brady and Weil 2010; Marschner 1991).
Soil Eh-pH, nutrient solubility, and assimilation by plants
Eh and pH are factors that strongly influence the mobility of many nutrients in complex chemical and biological environments (Gambrell and Patrick 1978; Laanbroek 1990). Based on thermodynamic laws, Eh-pH diagrams -- known as Pourbaix diagrams (Pourbaix 1945) -- represent stability areas of the various chemical forms of an element in a solution as a composite function of Eh and pH. Among the reactions that are possible thermodynamically, those that predominate at any given point in time are determined by redox kinetics (Chadwick and Chorover 2001). Therefore, the complex kinetics of oxidation-reduction processes in heterogeneous and changing soils should be considered carefully (Sparks 2001). These kinetics are also influenced by Eh and pH as they have an impact on microbial activity, which catalyzes these reactions (Fenchel et al. 1998).
Conversely, Eh and pH are influenced by the various elements composing the soil, especially those having a high amplitude in their redox numbers, such as N, P or S, and those at high concentrations such as Fe.
Nitrogen
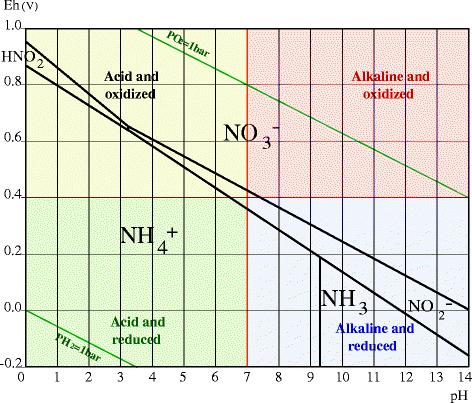
The nitrogen cycle is related to Eh and pH as shown by the Pourbaix diagram representing the predominant areas for the various forms of N in a water solution according to these parameters (Figure 1). In oxidized conditions (Eh > 500 mV at pH 7), the thermodynamically stable form of N is NO -3 ions, while under reduced or moderately oxidized conditions (Eh < 400 mV at pH 7) and at a pH below 9.2, NH +4 ions will dominate.
Pourbaix diagram of nitrogen (N) representing the various forms of N in a 100 μM solution at 25 °C as a function of Eh (in V) and pH. Diagram adapted from MEDUSA Software. Puigdomenech 2009–2011
As both NO -3 and NH +4 are soluble, Eh and pH mainly influence the form under which N is assimilated by plants. The form of N assimilated by plants also has a marked effect on cellular regulation of pH (Marschner 1995). Furthermore, as plants use NH +4 to synthesize proteins, the assimilation of NO -3 -N induces a considerable energy cost for the plant to reduce NO -3 -N to NH +4 -N (Marschner 1995). In addition, as NO -3 is highly soluble, there is a risk of loss, and pollution. Finally, the form of nitrogen assimilated by plants has a marked effect on rhizospheric pH and on the assimilation by plants of other cations and anions (Hinsinger et al. 2003; Marschner 1995).
Phosphorus
Under field conditions, plant roots absorb inorganic phosphorus dissolved in the soil solution, mainly as monovalent phosphate ions H2PO -4 which can much more easily be transported through the plasma membrane than divalent phosphate ions HPO 2-4 (Hinsinger et al. 2003). H2PO -4 ions dominate in acid soils while HPO 2-4 ions are by far the dominant species in alkaline soils. However, root apoplastic pH is normally less than 6, and the major form of P taken up across the membrane by plants in high-pH soils would still be H2PO -4 (Hinsinger et al. 2003). Furthermore, rhizosphere acidification as related to proton efflux from roots is well known as a response of many plant species to phosphorus deficiencies (Hinsinger et al. 2009).
Eh and pH also indirectly affect P availability by affecting the solubility of metal ions as Mn, Al and Fe oxides and hydroxides or of CaCO3; these bind to or adsorb phosphate ions and make them unavailable to plants (Brady and Weil 2010; Kemmou et al. 2006; Phillips 1998; Sallade and Sims 1997; Vadas and Sims 1998).
In addition, the H2PO -4 / HPO 2-4 system is known to play a prominent role in the buffering of cytosolic pH (Gerendas and Schurr 1999; Hinsinger et al. 2003).
Sulfur
Sulfur deficiency is rare in agriculture, especially in aerobic conditions. Under normal Eh-pH field conditions, the thermodynamically stable form of sulfur is sulfate SO 2-4 (Figure 2), which is transported and easily assimilated by plants. As the reduction of SO 2-4 to H2S occurs only at a low Eh and pH (around −100 mV at pH 6), the direct impact of Eh-pH on sulfur solubility is limited to waterlogged or submerged fields. In these fields, especially when they are rich in organic matter, a very low Eh leads to SO 2-4 reduction into H2S, which is highly toxic to plants (Ponnamperuma 1972). At a higher Eh and pH, sulfur availability is also indirectly affected, as Eh and pH influence the sulfate sorption capacity of the soil (Lefroy et al. 1993).
Pourbaix diagram of sulfur (S) representing the various forms of S in a 100 μM solution at 25 °C as a function of Eh (in V) and pH. Diagram adapted from MEDUSA Software. Puigdomenech 2009–2011
Conversely, in natural environments sulfur is one of the main determinants of the Eh-pH characteristics (Baas Becking et al. 1960). Furthermore, oxidation of reduced sulfur (H2S or FeS2), most of the time by autotrophic bacteria such as Thiobacillus spp., leads to the production of sulfuric acid and, as a consequence, to acidification (Dent 1993).
Iron
The solubility of iron is strongly influenced by both Eh and pH. Iron is absorbed by plants in its soluble form as ferrous iron (Fe2+). High concentrations of ferrous iron in the soil solution are observed at a low Eh, with a rapid decrease when the Eh rises above + 350 mV (at pH 5), in relation to the formation of iron oxides and hydroxides (Frohne et al. 2011). Ferrous iron toxicity is frequent at a low Eh and pH, and conversely, iron deficiency can occur at a high Eh and pH (Tanaka et al. 1966).
In natural environments, iron strongly influences the Eh-pH characteristics (Baas Becking et al. 1960), and iron reduction–oxidation delineates an important redox threshold in pedogenesis (Chadwick and Chorover 2001).
Potassium, sodium, and aluminum
Potassium, sodium, and aluminum solubility is not directly affected by Eh because these elements have only one possible redox number, respectively + I in K+ and Na+ and + III in Al3+, and they cannot exchange electrons.
Potassium availability is mainly related to soil pH, and to clay content and type. An increase in pH increases potassium fixation as it makes it easier for K+ ions to move closer to the colloidal surfaces. As they move closer, K+ ions are more susceptible to fixation by 2:1 clays, which fix potassium very readily and in large quantities (Brady and Weil 2010).
Sodic soils have high pH as H+ ions are adsorbed on clay micelles in place of Na+, which results in increased OH- concentration. Sodium Exchangeable Percentage (SEP), defined as Exchangeable Na/Cation Exchange Capacity, is positively correlated to soil pH (Abrol et al. 1988). Excess exchangeable sodium in sodic soils has a marked influence on the physical soil properties, leading to breakdown of soil aggregates, lowering the permeability of the soil to air and water (Abrol et al. 1988), and altering the soil's Eh. High pH induced by an excess of exchangeable sodium also indirectly affects other nutrient concentrations, as for P, Ca, Mg, Mn or Zn (Abrol et al. 1988).
Aluminum solubility is mainly governed by soil pH, but also by soil organic matter and clay content. Exchangeable aluminum rapidly increases when pH(KCl) decreases below 4.3. Aluminum plays a major role in soil acidity. The water molecules ligate to the aluminum ions forming Al(OH2) 3+6 ions, favoring water dissociation which produces protons (Manahan 2001). A single Al3+ can release up to three H+ (Brady and Weil 2010).
Micronutrients
The availability of several micronutrients, such as Mn, Cu or Zn, is strongly influenced by soil Eh and pH. There is evidence for direct or indirect biological alterations in the availability, solubility, or oxidation-reduction state of Mn, Zn, Cu, Mo, Co, Si, Ni, and various others (Reddy et al. 1986). For instance, the Pourbaix diagram for manganese (Figure 3) shows that solubilization as Mn2+ ions is a function of both Eh and pH. In alkaline soil, Mn deficiency can occur under aerobic conditions. A decrease in Eh corresponds to higher Mn bioavailability (Schwab and Lindsay 1982), and Mn toxicity is quite common in association with a low soil pH (Brady and Weil 2010).
Pourbaix diagram of manganese (Mn) representing the various forms of Mn in a 100 μM solution at 25 °C as a function of Eh (in V) and pH. Diagram adapted from MEDUSA Software. Puigdomenech 2009–2011
Similarly, Pourbaix diagrams for various micronutrients (not shown) indicate that deficiency is more likely: (i) at a high pH and/or low Eh for Cu; (ii) at a high pH for Zn; (iii) at a very low Eh or a high pH for Ni; and iv) at a low Eh and pH for Mo. This is confirmed by field observations of Cu and Ni concentration, which increases in the soil solution with a rising Eh (Frohne et al. 2011).
Plant impacts on soil Eh and pH
Plants can quite dramatically alter the Eh and pH of their rhizosphere (Hartmann et al. 2009). Plant roots create conditions allowing development of a unique microbial community in the rhizosphere, to such an extent that evolution has shaped soil life to adapt to this specific ecological niche (Hartmann et al. 2009; Lambers et al. 2009; Sanon et al. 2009). This alteration can be a direct effect of root exudation or an indirect effect through preferential development of specific microorganisms which also alter the Eh and pH.
Roots of plants adapted to highly-reduced environments obtain their essential oxygen through a system of air-filled intercellular space (Flessa and Fischer 1992a). Wetland plants, such as rice, have the ability to raise the Eh in their rhizosphere, especially by oxygen transport through aerenchyma (Evans 2004; Gilbert and Frenzel 1998; Gotoh and Tai 1957). This ability to raise the Eh in their rhizosphere protects plants against phytotoxic concentrations of reducing substances. It is related to the nutritional state of the plant. For instance, K application on K-deficient soils in paddy fields leads to an increase in the oxidizing power of rice roots, an increase in soil Eh and a decrease in active reducing substances and ferrous iron, and a decrease in the number of oxygen-consuming microorganisms (Chen et al. 1997).
In aerobic conditions, the rhizosphere of various dicotyledonous cultivated plants (Pisum sativum, Vicia sativa, Helianthus annuus) showed a decrease in Eh close to the root tip, probably due to the release of reducing exudates (Flessa and Fischer 1992b).
The pH in the rhizosphere is also altered by plant roots and soil microbes. Rhizosphere pH has been reported to be up to 1–2 pH units below or above the bulk soil pH, and differences were detected up to 2–3 mm from the root surface (Chaignon et al. 2002; Hinsinger et al. 2009; Hinsinger et al. 2003; Youssef and Chino 1989). Miller et al. (1991a) in a study on barley (Hordeum vulgare) and white clover (Trifolium repens) showed that the rhizosphere at the root tip is alkaline relative to that further from the tip. The form under which plants absorb nutrients substantially affects rhizospheric pH, especially for nitrogen as NH +4 or NO -3 ions amount to 80 % of the total anions and cations assimilated by plants (Marschner 1995).
Dramatic changes in pH can occur as a consequence of the microbially-mediated oxidation of nitrogen (Hinsinger et al. 2009). This is explained by: (i) the necessary release by plant roots of an H+ ion when they absorb a NH +4 ion to counterbalance the corresponding excess of positive charges (Hinsinger et al. 2003; Raven and Smith 1976); (ii) conversely, the release of OH- ions when plants absorb NO -3 ions to counterbalance the corresponding excess of negative charges, the excess OH- being also partly neutralized by the 'biochemical pH-stat' (Raven 1986; Raven and Smith 1976). In addition to roots, many soil microbes, such as ectomycorrhizal and saprophytic or pathogenic fungi, can produce organic acids and acidify the rhizosphere (Hinsinger et al. 2009).
However, modifying the Eh and pH of the rhizosphere has a cost for plants. In annual plant species, 30-60 % of the photosynthetically-fixed carbon is translocated to the roots, and a large proportion of that carbon (up to 70 %) can be released into the rhizosphere (Neumann and Römheld 2000). For instance, cereals (wheat and barley) transfer 20-30 % of total assimilated C into the soil, and pasture plants translocate about 30-50 % of their assimilates below ground (Kuzyakov and Domanski 2000). Thus, rhizodeposits, the organic compounds released by plant roots under various forms, amount to 20 to 50 % of the total photosynthetic production, but can be as high as 80 % (Gobat et al. 1998).
Beside their short-term effect in the rhizosphere, plants also have an impact on soil Eh and pH in the long term, as they are the main source of organic matter in the soil. Whether or not their biomass is returned to the soil has a large and long-term effect on the structure and functioning of soil systems.
Soil Eh and pH and soil organic matter
Organic matter is one of the main factors affecting soil Eh (Oglesby 1997). Bioavailable organic matter as an electron reservoir constitutes the bulk of the soil's reduction capacity (Chadwick and Chorover 2001; Lovley et al. 1998). Organic matter can be partly, and reversibly, reduced by microorganisms, and it plays the role of electron shuttle, i.e. a mobile carrier of electrons (Lovley et al. 1998). Organic matter is the most prolific source of electrons in soil and during decay may be looked upon as an electron-pump, supplying electrons to more oxidized species in the soil (Chesworth 2004).
An increase in soil organic matter leads to a lowering of soil Eh: in soils rich in easily decomposable organic matter, oxidation processes consume large amounts of oxygen, which leads to the formation of organic compounds with reducing properties (Lovley et al. 1998). Fresh organic matter is the most reduced fraction in soil, hence the most thermodynamically unstable, followed by necromass and most of the soil organic matter, with organic quinone molecules being the most resistant to oxidation (Macías and Camps Arbestain 2010).
Fresh straw was reported to have an Eh around +150 mV (at pH 5.5 to 6). During composting the Eh evolved from 0 (at pH 7.7) at the beginning of the process to +300- + 400 mV at the end of composting (Miller et al. 1991b). To summarize, the quality and the quantity of organic matter largely determine soil Eh and its buffering capacity.
Organic matter is also one of the main factors buffering soil pH (Magdoff and Bartlett 1984). It contributes to the development of neutral to slightly acid soil pH (Brady and Weil 2010). At a low pH, soil organic matter forms complexes with Al, which is a major pH-buffering process in soils (Skyllberg et al. 2001). At a high pH, organic matter contributes to acidification through the formation of soluble complexes with non-acid cations such as calcium or magnesium, which are easily lost by leaching (Brady and Weil 2010).
On the other hand, soil Eh and pH are some of the main factors regulating the speed and intensity of humification processes (Reddy et al. 1986; Rusanov and Anilova 2009). Organic matter degradation rates are fastest under oxidizing conditions, in the presence of free O2 (Macías and Camps Arbestain 2010). In the absence of free O2 or inorganic oxidants as NO -3 , Mn4+, Fe3+ and SO 2-4 , fermentation processes take place in which organic molecules are utilized as electron acceptors (Reddy et al. 1986; Ugwuegbu et al. 2001).
In the global carbon cycle, the length of time spent by the carbon in the soil as solid organic matter is a function of Eh. Organic matter represents a residence time for carbon as short as a year in oxidized soils, and of thousands of years in highly reduced conditions (Chesworth 2004). The rate of organic matter decomposition is also influenced by three parameters related to soil Eh and pH: (i) the type of microbial metabolism; (ii) the bacterial efficiency; and (iii) the capacity of the soil system to supply electron acceptors (Reddy et al. 1986).
Soil Eh and pH and soil genesis
The Eh and pH of the soil solution are key factors influencing the trajectory of soil genesis (Chadwick and Chorover 2001; Chesworth 2004; Chesworth et al. 2006; Chesworth and Macias 2004). This trajectory can be represented on Eh-pH diagrams (Figure 4). The chemical evolution of soils is essentially determined by fluxes of protons and electrons. The electron flux takes place between organic matter as the major source, and atmospheric oxygen as the principal sink (Chesworth and Macias 2004).
The combined effect of proton and electron pumping is to confine most soils within an envelope in Eh-pH space, with three salients or dimensions, i.e. acidity, alkalinity, and hydromorphism, the permanent or temporary state of water saturation in the soil. The acid salient represents the path of evolution of three major types of soil genesis: podzolization, ferralitization, and andosolization (Chesworth and Macias 2004). Calcareous, sodic and saline soils develop along the alkaline salient, while peat soils develop along the hydromorphic salient, with excess moisture leading to suppression of aerobic factors in soil-building.
Such Eh-pH diagrams are particularly interesting for studying acid sulfate soils. In these soils, changes in Eh dramatically affect pH as they contain pyrite (FeS2), which upon oxidation produces large amounts of sulfuric acid. Potential acid sulfate soils, with pyrite, are found in reduced conditions. Upon oxidation (> 50 mV at pH 4), actual acid sulfate soils develop as pyrite oxidation leads to strong acidification (Dent 1993; Husson et al. 2000b).
Eh, pH, and environmental issues
In general, the concentration of heavy metals and pollutant metalloids in the soil solution is influenced by the combination of soil Eh and pH. Cadmium (Cd) and lead (Pb) concentrations are low at a low Eh and rise when the Eh increases, which can be attributed to interactions with dissolved organic carbon and manganese and precipitations such as sulfides (Frohne et al. 2011; Stepniewska et al. 2009). Cd solubility decreases with organic matter inputs because of the induced decrease in Eh and the increase in pH (Kashem and Singh 2001). Conversely, the concentrations of the various forms of arsenic (As) and antimony (Sb) sharply decrease when the soil Eh increases, indicating that low Eh promotes the mobility of these compounds (Frohne et al. 2011).
In its oxidized form (Hg2+ ions), mercury is highly soluble but easily adsorbed by organic matter. In the presence of sulfur and at a low Eh (<−100 mV), Hg can precipitate as insoluble HgS. Hg is highly soluble and toxic as methylmercury, which is only produced by methylating microorganisms, especially Clostridium spp., which develop within a given Eh-pH range, with Eh between −400 mV and +100 mV (Billen 1973).
Furthermore, oxidation-reduction reactions control the transformation and reactivity of Fe and Mn oxides, which have a high capacity to adsorb heavy metals and pollutant metalloids and are major sinks for these pollutants (Huang and Germida 2002).
Oxidation-reduction reactions largely drive bioremediation processes. For instance, humic acids are electron acceptors enabling anaerobic microbial oxidation of vinyl chloride and dichloroethene (Bradley et al. 1998). Atrazine biotransformation is also oxidation, enhanced by Mn and inhibited by antioxidants (Masaphy et al. 1996); this biotransformation is extremely rapid in oxidized soils (Eh > 392 mV). It is much slower in reduced conditions (Eh < 169 mV), such as in wetland soils where atrazine can persist for months (DeLaune et al. 1997).
Eh can be used as an indicator of soil health during remediation processes and as an indicator of the rate of remediation of a soil contaminated with polycyclic aromatic hydrocarbons (Owabor and Obahiagbon 2009; Ugwuegbu et al. 2001). The redox poising capacity can also be used as a tool for assessing long-term effects in natural attenuation/intrinsic remediation, based on processes such as biological degradation, dispersion, dilution, sorption, evaporation, and/or chemical and biochemical stabilization of pollutants (Von de Kammer et al. 2000).
In most soils, production or consumption of the three major greenhouse gases (nitrous oxide N2O, methane CH4, carbon dioxide CO2) in oxidation-reduction reactions is regulated by interactions between the soil Eh, the carbon source, and electron acceptors such as O2, Mn4+, Fe3+, nitrate, sulfate or hydrogen (Li 2007). Other factors such as temperature, moisture, and pH can also affect the biochemical or geochemical reactions related to soil N2O, CH4 or CO2 emissions (Li 2007).
The CH4 formation process and emission are controlled by Eh and pH (Wang et al. 1993). N2O emission is correlated with soil Eh (Kralova et al. 1992; Masscheleyn et al. 1993; Wlodarczyk et al. 2003; Yu and Patrick 2003), and with soil pH as pH influences the three most important processes that generate N2O and N2: nitrification, denitrification, and dissimilatory NO -3 reduction to NH +4 (Simek and Cooper 2002). Eh and pH also interact in determining N2O and CH4 emission: the Eh range with minimum N2O and CH4 production shifts to lower values of the Eh scale when pH increases (Yu and Patrick 2003).
Soil Eh-pH and agronomy
Eh and pH measurement
One of the major difficulties in using Eh and pH in agronomy lies in the measurement of these parameters, especially the Eh in aerobic soils. Literature on the subject is sometimes controversial, as shown by numerous reviews (Bartlett and James 1993; De Mars and Wassen 1999; Fiedler et al. 2007; Gantimurov 1969; Greenland and Hayes 1981; Kaurichev and Orlov 1982; Kovda 1973; Rabenhorst et al. 2009; Snakin et al. 2001; Unger et al. 2008; Zakharievsky 1967).
These reviews reported two main problems: (i) the quality and the reliability of the equipment, especially for Eh, as different types of electrodes have been developed and as leaks or polarization of the electrodes can occur and distort the measurements; and (ii) the high spatial and temporal variability of soil Eh and pH, and consequently, the limited reliability of sampling and analysis methods, which needs to be carefully addressed.
Protocols are needed that ensure the reliability of measurements and their sound interpretation, on the appropriate scale. Major progress has been made in the development of Eh electrodes since Remezov measured the Eh in soil for the first time in 1929. Recommendations have been developed for the selection and utilization of Eh electrodes (Fiedler et al. 2007; Snakin et al. 2001). However, careful attention should still be given to minimize the risk of measurement distortion and proper characterization of the variability of Eh and pH is still needed to standardize sampling and measuring methods on various scales.
Furthermore, Eh and pH are not independent. For example, in anaerobic soils, soil Eh increases with acidification, and decreases with alkalinization (Van Breemen 1987). On this basis, the pH at which this Eh has been measured should be systematically mentioned. A striking feature of this Eh literature review was that studies only occasionally associate the Eh with the pH, and they rarely pay attention to the possible interaction between Eh and pH in the processes studied. Under such conditions, comparisons between studies are difficult, which partly explains why Eh has not received sufficient attention in agronomic studies.
Finally, Eh does not uniquely reflect electron activity, as it is also linked to proton activity (Fougerousse 1996; Orszagh 1992). For a more effective characterization of oxidation-reduction, the chemical notion of rH2 has been used in various disciplines linked to living organisms, including biological physics (Deribere 1949; Fougerousse 1996; Huybrechts 1939; Orszagh 1992; Rabotnova and Schwartz 1962; Vincent 1956; Vlès 1927; 1929). rH2 is defined in analogy with pH, as –log[H2] , where [H2] is the thermodynamic activity of molecular hydrogen that would be formed following electron exchanges between water and solute species (Orszagh 1992). In agronomy, some authors proposed using the pe concept (electronic potential), defined as –log[e-] where [e-] is the hypothetical activity of electrons (Lindsay 1979; Sillen and Martell 1964; Sposito 1989; Stumm and Morgan 1981; Truesdell 1969). pe + pH was also proposed as a redox parameter for soils (Lindsay 1983). The most appropriate way to characterize oxidation-reduction is still a question which needs to be addressed.
The application of Eh-pH diagrams
Since their first application to the earth sciences by Krumbein and Garrels (1952), Pourbaix diagrams have been used in other sciences such as soil chemistry, geochemistry or hydrobiology. In geochemistry, a study on over 6,200 pairs of measurements showed two interesting results: (i) the Eh and pH of an environment such as soil or water can be used to characterize that environment in many ways; and (ii) the Eh-pH characteristics of natural environments are determined mainly by photosynthesis, respiration, and redox couples of iron and sulfur (Baas Becking et al. 1960).
In agronomy, Eh-pH diagrams are classically used for studies of submerged soils, especially paddy fields. In such rapidly changing environments, Eh and pH are essential parameters, and Pourbaix diagrams are useful tools for understanding their chemistry (Ponnamperuma 1972). In contrast, studies of Eh in aerobic soils are rare.
Despite their scarcity, studies of Eh in aerobic soil conditions have shown that Eh could be a useful agronomic tool for characterizing field conditions. In one of the few studies, Snakin et al. (2001) used the Eh and pH of the soil liquid phase as ecological indicators. Using Eh-pH diagrams, these authors were able to separate environmental entities into various groups by: (i) ecosystem type: agricultural, grassland, and forest communities; (ii) vegetation type: coniferous, broad-leaved, meadow, meadow-steppe, and steppe vegetation; and (iii) soil type: podzolic, grey forest, chernozem, and chestnut soils (separately for agricultural and natural communities). They also used Eh-pH diagrams to characterize the distance between agricultural soils and the initial virgin milieu, represented by a vector.
Impacts of agricultural practices on soil Eh and pH
Four main agricultural practices can affect soil Eh and pH: application of soil amendments (organic or chemicals), water management, land preparation, and crop rotation. Amendments with lime and/or organic matter are commonly used to alter soil pH (Brady and Weil 2010; Whalen et al. 2000). Various methods have been proposed to correct soil pH by liming, especially in response to Al toxicity (Dietzel et al. 2009). Herbel et al. (2007) proposed to poise soil Eh using chemicals such as NaNO3 or Mn oxyhydroxides.
In addition to practices specifically designed to correct undesirable soil chemical characteristics, the use of agrochemicals has an influence on the Eh and pH. For instance, fertilizers such as superphosphate are acidifying, and others such as Thomas slag are alkalinizing. Oxidation of urea or ammonium sulfate by microbes produces strong inorganic acids (Brady and Weil 2010). Herbicides also induce oxidative stress (Blokhina et al. 2003): for instance, paraquat (1,1'-dimethyl-4,4'-bipyridinium dichloride) is an oxidant herbicide, operating by stimulation of superoxide production in organisms (Cocheme and Murphy 2009).
Irrigation and drainage greatly affect soil Eh and pH. Soil saturation reduces oxygen diffusion through soils as the diffusion coefficient of oxygen through solution is about 10,000 times slower than diffusion in the gaseous phase (Stolzy and Letey 1964). When microorganisms consume oxygen for respiration, this leads to a rapid decrease in soil Eh (Flessa and Fischer 1992a; Lambers et al. 2008). The pH tends toward neutral conditions under continuous flooding: the pH of acidic soils will increase whereas the pH of alkaline soils decreases (Glinski and Stepniewski 1985; Ponnamperuma 1972).
Land preparation also affects soil Eh and pH, mainly because by altering soil structure. Bulk density and aggregate size greatly influence soil water desorption and the depth of soil to which O2 could diffuse (Grable and Siemer 1967). Soil management affects pores' continuity and the hydraulic, gas, and heat fluxes (Horn and Peth 2009). Reduction in the number of conducting coarse pores leads to anoxic conditions (Horn and Peth 2009). On grey soils, Snakin et al. (2001) measured a significant Eh gradient between horizons in natural forests, but not in cultivated fields. Using an Eh-pH diagram, these authors could separate agricultural land from grassland and from forest communities. In four soils, from sand to loam, Czyz (2004) showed that soil compaction decreases soil aeration and lowers the Eh. Soil tillage modifies soil aeration and affects the Eh in the surface soil horizon, but also below the tilled zone, in interaction with rainfall (Clay et al. 1992; Olness et al. 1989).
The type of plants cultivated and their sequence in the crop rotation influences soil Eh and pH for two reasons: (i) plants and their associated microorganisms play a crucial role in the formation and alteration of soil (Lambers et al. 2009); and (ii) plant biomass production and inputs to the soil influence soil organic matter content, which buffers the Eh and pH of the bulk soil (Paustian et al. 1997). In the surface horizon (0–30 cm) of an aerobic soil, Bohrerova et al. (2004) measured significant soil Eh differences between cropping systems, with a higher Eh on an alfalfa/wheat rotation than on a sugar beet/barley rotation. Furthermore, by creating a different leaf pH from that of the soil they grow in, and given the cross-species correspondence of green leaf pH and leaf litter pH, plant species can alter the pH of the soil they grow in (Cornelissen et al. 2011).
Possible modification of rhizosphere Eh and pH by plants and microorganisms towards an optimum physiological level
It has been recognized for many years that plant energy use and metabolism contributes to internal pH homeostasis (Kurkdjian and Guern 1989; Rengel 2002). A recent ecological study of 23 herbaceous plant species showed that leaf pH is independent of bulk soil pH and suggested that tissue pH itself is tightly controlled for any given species, because of its direct or indirect functions in the plant (Cornelissen et al. 2011). From the perspective of this review, I can put forward a wider hypothesis: since physiological processes in plant cells only operate properly within a fairly narrow and specific Eh-pH range, plants develop mechanisms to ensure their cells' homeostasis at an optimum Eh-pH level. Alteration of the Eh and pH conditions in their near environment, i.e., in the rhizosphere, is part of the complex interacting processes that have evolved to ensure plants' internal Eh and pH homeostasis and, consequently, their cell physiological processes.
The available literature already indicates that the Eh and pH values in the rhizosphere converge toward 'ideal' Eh-pH values, especially around root tips. For instance, in reduced conditions, rice roots were able to raise the Eh from +120 mV in bulk soil to +420 mV at the root surface, influencing the soil Eh up to 4 mm from the root surface (Flessa and Fischer 1992a). Conversely, in oxidized conditions, the root tips of soil-grown faba bean (Vicia faba L.) induced an Eh decrease from +700 mV to +380 mV when they reached the microelectrode (Fischer et al. 1989). For pH also, it is noteworthy that plants behave as if their roots are avoiding either too low or too high a pH level around themselves (Hinsinger et al. 2003). A pH rise in the rhizosphere as compared to bulk soil can be up to 2 pH units in acid soils (Hinsinger et al. 2003). Conversely, in alkaline soils, Chaignon et al. (2002) reported a decrease in rhizosphere pH to 6.9.
Unfortunately, Eh and pH are rarely studied in association, and information on initial soil Eh and pH is never given in studies on root exudation and respiration. As a result, this hypothesis can neither be confirmed nor rejected from the available literature: but it certainly needs to be tested.
Issues for agronomists
Characterization of an 'ideal' soil by its Eh and pH
I propose that Eh and pH can be used, in interaction, as relevant primary parameters to characterize a soil and define what is an 'ideal' soil for a given crop.
The concept of an 'ideal' soil is not new. Various disciplines related to agronomy have proposed to characterize an 'ideal' soil either (i) according to individual parameters such as base-cation saturation ratio (Kopittke and Menzies 2007), moisture (Keen 1924), and capillary pull (Hackett and Strettan 1928), or (ii) through integrated parameters (De Orellana and Pilatti 1999; Janssen and de Willigen 2006; Pilatti and de Orellana 2000).
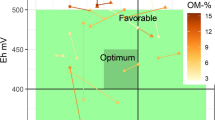
This review makes it possible to estimate an Eh-pH domain of optimum conditions for plant growth (Figure 5). The optimum pH for most cultivated plants is 6.5 to 7, and rather favorable conditions for plant growth are found between 5.5 and 8. The optimum Eh for plant growth is probably in the range of +400 to +450 mV. Below +350 mV, plant growth rapidly decreases. The upper Eh limit is difficult to determine with available information, but also it may vary with plant species and the soil pH and other characteristics. However, I believe that at a pH of 6.5 to 7, an Eh above +450 mV to +500 mV is unfavorable, with a risk of mineral deficiency (P, Mn, Fe), heavy metal toxicity (Cd, Pb), and pathogen development.
These optimum Eh-pH values correspond to the transition between the two major forms of N in the soil, NO -3 and NH +4 (Figure 1). It is well known that for most plants, the highest growth is obtained with mixed NO -3 and NH +4 nutrition (Marschner 1995). These Eh-pH values also correspond to a high availability of all major plant nutrients and micronutrients (N, P, Mg, Mn, etc.), and to a minimum risk of toxicity by heavy metals, metalloids, Al, or Fe. A soil with such 'ideal' Eh-pH characteristics would be in accordance with optimum plant physiological conditions and would provide all nutrients in a readily available form, with a low risk of mineral toxicity. This 'ideal' soil would also provide favorable conditions for development of 'useful' soil microorganisms, and unfavorable conditions for pathogens.
In such an 'ideal' soil, energy use efficiency is at a maximum to ensure cell homeostasis. Most photosynthetic products can thus be used for the metabolism and growth of plants and associated microorganisms. Plant production is optimized, given that the resulting high biomass production generates C fluxes in the soil, more humus formation, and development of useful microorganisms. These fluxes and microorganisms contribute to the buffering of the soil's Eh and pH at favorable levels. The soil/plant/microorganism system is thus efficient and stable.
It can be expected that the more distant that soil Eh-pH is from an optimum physiological Eh-pH, the higher will be the energy cost for plants to ensure their cells' homeostasis. When soil Eh-pH is very different from the optimum physiological Eh-pH, the capacities of the plant to maintain the soil Eh and pH at a favorable level can be overloaded.
Physiological dysfunctions in reduced environments have been widely recognized and studied, especially for rice crops (Ponnamperuma 1972; Yoshida 1981). What has less been acknowledged is that two processes sustain and increase these reduced conditions in a vicious circle: (i) very slow mineralization in the absence of oxygen and under unfavorable conditions for the microorganisms involved in mineralization processes; and (ii) buffering of the Eh at low levels by anaerobic microorganisms which preferentially develop in these conditions.
Similarly, above an Eh threshold estimated at + 450 mV to + 500 mV, four processes sustain and increase the Eh in a reinforcing circle: (i) low energy efficiency at high Eh leads to slow plant growth and low biomass production; (ii) consequently, leaf area and solar energy interception are limited, and the reduction capacity of the plant is kept low; (iii) low biomass production also leads to losses in organic matter, all the more so as highly oxidized conditions favor mineralization (Chesworth 2004; Macías and Camps Arbestain 2010); and finally (iv) low organic matter content in the soil leads to a low poising capacity and a rise in Eh.
Use of resistivity to characterize a soil
This review makes clear the importance of Eh, an electrical potential, in soil/plant/microorganism systems, in accordance with the perception of Szent-Gyorgyi (1960) that “what drives life is a little electric current.” It suggests that soil/plant/microorganism functioning could be regarded as an electrical circuit.
In physics, the functioning of an electrical circuit cannot be described using only its voltage (V, in Volts). Its resistance (R, in Ohms) is also needed. Ohm’s law (I = V/R) allows to calculate the intensity (I, in Amperes), an essential parameter characterizing the flow of electrons in a conductor or ions in an electrolyte. An Ohm’s law analogy is used in many situations in biology and ecology as for example Fick’s law of diffusion.
By analogy, in soil/plant/microorganism systems the electrical resistivity can be regarded as the resistance in Ohm’s law. Electrical conductivity (EC), the inverse of the resistivity, can be regarded as the diffusivity in Fick’s law.
Soil electrical resistivity has been used for over a century to characterize a milieu, and its use has intensified since the 1970’s. Today, it is used in precision agriculture as a very attractive indicator to characterize soil (Samouëlian et al. 2005). This non-invasive measuring technique enables researchers to characterize various soil properties, such as the cation exchange capacity (CEC), salinity, nutrients, residual humidity and preferred water flows, soil texture and properties related to texture (sand layers, impermeable clay layers, etc.), bulk density, and compaction areas or organic matter (Paillet et al. 2010; Samouëlian et al. 2005). For instance, soil resistivity decreases when bulk density increases, especially when soil water content is low (Richard et al. 2006; Seladji et al. 2010). Electrical resistivity is also used to estimate the level of soil weathering (Son et al. 2010).
This suggests that using soil electrical resistivity in addition to Eh and pH could be very useful. The description and the understanding of the functioning of soil/plant/microorganism systems could be improved by calculating derived parameters such as intensity. In animal production, the use of resistivity to calculate an intensity proved to be successful to analyze farm animals by analogy with electrical circuits (Aneshansley and Gorewit 1991; Rigalma et al. 2009). In aquaculture, it is appreciated that Eh, pH, and resistivity are strong drivers for fish, shellfish, or oyster production. They have been integrated in a software (Vortex 2008–2011, designed by the French firm Idee-aquaculture) used on a large scale to pilot aquaculture ponds. This raises two questions for agronomists: (i) is such an analogy valid for plant production? and (ii) do Eh, pH, and resistivity capture the essential information needed to characterize systems as complex as soil/plant/microorganism systems?
An Eh-pH-resistivity perspective: new insight in agronomy
This integrated review had brought together converging evidence from several disciplines that Eh, pH, and resistivity are fundamental parameters that can be integrated into a revealing conceptual model of soil/plant/microorganism system functioning. If this model is validated, and it becomes accepted that an 'ideal' soil can be defined by Eh, pH, and resistivity for a given crop, this analytical perspective could open up avenues for new investigations and could generate scientific advances in various disciplines connected to agronomy.
For instance, this framework could be used to analyze Genotype x Environment x Management (GxExM) interactions. Under this perspective, GxExM interactions could be explained by assessing varietal differences under optimum physiological Eh-pH, considering their ability to sustain homeostasis in relation with the initial bulk soil Eh-pH and management practices.
This conceptual model of soil/plant/microorganism systems could also provide a sound framework to explain the high variability of rhizodeposition found in the literature: it predicts that rhizodeposition should be a function of initial bulk soil Eh-pH and of management practices, which are not considered so far in studies on rhizodeposition.
Furthermore, this conceptual model proposes some new insights on the role and importance of soil organic matter, especially in upland agriculture: organic matter poises the Eh and pH at favorable levels, which reduce the energy cost for plants to ensure their Eh-pH homeostasis. This could also explain the variability in fertilizer efficiency and the phenomenon of hysteresis during soil productivity restoration (Tittonell et al. 2008). Low organic matter content, and thus oxidized soil conditions, leads to low fertilizer efficiency in the short term because plants have to release a large share of their photosynthetic production as root exudates to adjust the Eh in the rhizosphere and ensure cell homeostasis.
This integrative perspective on the importance of the Eh in soil/plant/microorganism systems may also help explain both: (i) the success of the Green Revolution in Asia under the reduced conditions of flooded paddy fields because the oxidizing practices (land preparation and soil aeration, mineral fertilization, chemical pesticide applications, etc.) rapidly raised Eh to a more favorable level; and (ii) the trend of declining yields in long-term experiments (Ladha et al. 2003) because the continued oxidizing practices over long time periods may generate over-oxidation, leading to consequent yield decline. The good results obtained by SRI techniques (System of Rice Intensification) may also be explained by the maintenance of a favorable soil Eh-pH when alternating irrigation and drainage (Stoop et al. 2002).
Furthermore, this new perspective could help explain an increase in fungal diseases when pesticides are used, as reported for Fusarium sp. pathogens with glyphosate (Fernandez et al. 2009). Pesticide application leads to oxidative stress in plants, which may create favorable conditions for pathogenic fungi. This framework also supports the trophobiosis theory of Chaboussou (1985) that physiological dysfunction induced by hydric stress and/or application of chemical inputs favors insect or pathogens attack: these stresses are related to pH and/or to oxidative stresses as most chemical fertilizers and pesticides are oxidants (Bressy 1996).
In addition, this new framework for analysis of soil/plant/microorganism systems could help in bridging the gap between chemistry-physics and biology and may be a useful tool for the integration between disciplines, which is needed for the development of new perspectives in agronomy (Jones et al. 2004). Sparks (2001), for instance, regards the studies of microbe-driven redox reactions and redox transformations of C, N, P, and S according to various redox limits as new frontiers of research at the interface between chemistry and biology.
Similarly, this new framework could help in transcending the constraints of scale. The lack of perspective across scales is a major limit for research, and major progress will be achieved if scientists are able to bridge across micro and macro scales, from the rhizosphere of individual roots to soils at field scale and larger (Hinsinger et al. 2009). In a systemic approach, an average value of a parameter for a system (studied at one particular scale) is determined by interactions of the various sub-systems composing that system (on a finer scale).
Understanding the structure and the origin of the variability of essential parameters such as Eh and pH can help for scale transfers and also for understanding processes across scales. For instance, understanding the structure and the function of cell compartmentalization for Eh and pH was crucial to understanding fundamental processes in cell physiology (Hansen et al. 2006; Scheibe et al. 2005). At another scale, Hinsinger et al. (2009) consider that it is urgent to improve the methodology for describing the physical architecture of the rhizosphere on a micro-scale and to express these findings to interpret the data in respect of their functional relevance.
At a considerably larger scale, understanding the structure and the origin of the tremendous soil variability in acid sulfate soils in Vietnam, in relation to Eh and pH, proved to be a major step for designing more efficient cropping systems (Husson et al. 2000a; Husson et al. 2000b). Furthermore, in environmental biotechnology, following electrons as they move through the ecosystem is regarded as the surest way of translating knowledge about structure and function into practice (Rittmann 2006).
Translating soil Eh, pH, and resistivity into production potential
Is it possible to translate the information provided by the three parameters Eh, pH, and resistivity of the soil into a production potential for plants, in relation to plant physiology? The production potential of a given crop is mainly determined by climatic factors such as temperature, water and sunlight, and by soil characteristics. I believe that Eh, pH, and resistivity can considerably improve the characterization of a milieu, and thus produce a useful evaluation of the production potential of a soil. However, a major challenge for agronomy will be to determine the optimum Eh-pH-resistivity of a soil, to maximize the production potential of a plant as a function of climate.
One major question remains regarding the relationship between these parameters and a production potential for a given soil. Interactions between the climate and these parameters have to be assessed, particularly temperature, as it affects the Eh, pH, resistivity and kinetics of biochemical reactions, and microorganism development probably has a major impact. Optimum soil Eh-pH-resistivity for plant production is very likely to be a function of temperature, and to vary according to climate, with rainfall affecting not just water but also oxygen availability in the soil.
Conclusions: Eh, pH, and resistivity as tools for designing and managing cropping systems
This framework and the improved soil characterization it provides could be a tool for modeling soil/plant/microorganism interactions. The definition of optimum ranges of soil Eh-pH-resistivity would help with the design and management of cropping systems. The creation of a cropping system could then be based on identifying plant species, microorganisms, and cropping practices that allow the development of favorable soil conditions, using this optimum soil Eh-pH-resistivity as a 'target' zone.
Insights from this analytical perspective could especially contribute to planning 'ecological intensification' of agriculture, which is defined as the use of biological regulation in agroecosystems both to achieve a high level of food production and to provide ecosystem services (Dore et al. 2011). For instance, Eh-pH-resistivity parameters might be used to develop rhizosphere-driven selection of microbes to improve the development and health of plants, which has been suggested as a major challenge for agronomy for the future of sustainable agriculture (Hartmann et al. 2009).
Such an approach has been rarely, and only very partially, used by agronomists to correct the soil Eh for various purposes. Carter (1980) used Eh assessments to pilot drainage for sugarcane production; Savich et al. (1980) proposed to use oxidants such as KMnO4, NaClO4 or Fe2O3 to regulate the Eh; Patsoukis and Georgiou (2007b) used sulfites and nitrites as cytotoxic oxidants to sustain fungi in their undifferentiated hyphal stage, in which they are more vulnerable to degradation by soil microorganisms; Blok et al. (2000) and Shinmura (2004) introduced easily decomposable organic material and flooded or covered the soil with airtight plastic to reduce the Eh and control Fusarium oxysporum and Rhizoctonia solani. Takehara et al. (2004) used allelopathic plants to lower the soil Eh and control soil-borne pathogens.
Such practices could be largely improved if Eh, pH, and resistivity were integrated to improve soil characterization and identify optimum conditions, which could be used as 'target' zones, as is done in aquaculture. Scientific development of such approaches will require intensive knowledge on specific optimum soil Eh-pH-resistivity values for plants and microorganisms, and on how the various species alter these parameters. If this proposed framework for soil/plant/microorganism systems is validated, a major challenge for agronomists of different disciplinary orientations will be to develop such knowledge further and translate it into sound agricultural practices.
References
Abrol IP, Yadav JSP, Massoud FI (1988) Salt-affected soils and their management. FAO Soils Bulletin 39, Rome
Ahmad S (1992) Biochemical defence of pro-oxidant plant allelochemicals by herbivorous insects. Biochem Syst Ecol 20:269–296
Alexander M (1964) Biochemical ecology of soil microorganisms. Annu Rev Microbiol 18:217–250
Altomare C, Norvell WA, Bjorkman T, Harman GE (1999) Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295–22. Appl Environ Microbiol 65:2926–2933
Aneshansley DJ, Gorewit RC (1991) Physiological and behavioral effects. In Effects of electrical voltage/current on farm animals How to detect and remedy problems. Lefcourt A M (eds). pp 1–23. Agriculture Handbook 696 US Department of Agriculture
Appel HM (1993) Phenolics in ecological interactions: the importance of oxidation. J Chem Ecol 19:1521–1552
Appel HM, Maines LW (1995) The influence of host plant on gut conditions of gypsy moth (Lymantria dispar) caterpillars. J Insect Physiol 41:241–246
Atkins PW, Jones L (1997) Chemistry: molecules, matter & change. W.H. Freeman and Co, New York
Baas Becking LGM, Kaplan IR, Moore D (1960) Limits of the natural environment in terms of pH and oxidation-reduction potentials. J Geol 68:243–284
Baier M, Dietz KJ (2005) Chloroplasts as source and target of cellular redox regulation: a discussion on chloroplast redox signals in the context of plant physiology. J Exp Bot 56:1449–1462
Bailly C, Bouteau HEM, Corbineau F (2008) Rôle de la signalisation par les espèces réactives de l'oxygène dans la germination et la levée de dormance des semences. J Soc Biol 202:241–248
Balakhnina TI, Bennicelli RP, Stepniewska Z, Stepniewski W, Fomina IR (2010) Oxidative damage and antioxidant defense system in leaves of Vicia faba major L. cv. Bartom during soil flooding and subsequent drainage. Plant Soil 327:293–301
Ball P (2008) Water as an active constituent in cell biology. Chem Rev 108:74–108
Bartlett RJ, James BR (1993) Redox chemistry of soils. Adv Agron 50:151–208
Bayliss NS (1956) The thermochemistry of biological nitrogen fixation. Aust J Biol Sci 9:364–370
Becker B, Holtgrefe S, Jung S, Wunrau C, Kandlbinder A, Baier M, Dietz KJ, Backhausen JE, Scheibe R (2006) Influence of the photoperiod on redox regulation and stress responses in Arabidopsis thaliana L. (Heynh.) plants under long- and short-day conditions. Planta 224:481–481
Best EJ, Samuel G (1936) The reaction of the viruses of tomato spotted wilt and tobacco mosaic to the pH value of media containing them. Ann Appl Biol 23:509–537
Billen G (1973) Etude de l'écométabolisme du mercure dans un milieu d'eau douce. Aquat Ecol 7:60–68
Bisio G, Bisio A (1998) Some thermodynamic remarks on photosynthetic energy conversion. Energ Convers Manage 39:741–748
Blankenship RE (2002) Molecular mechanisms of photosynthesis. Blackwell Science Ltd, Oxford
Blok WJ, Lamers JG, Termorshuizen AJ, Bollen GJ (2000) Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 90:253–259
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bohn HL (1971) Redox potentials. Soil Science 112:39–45
Bohrerova Z, Stralkova R, Podesvova J, Bohrer G, Pokorny E (2004) The relationship between redox potential and nitrification under different sequences of crop rotations. Soil Till Res 77:25–33
Bolwell GP, Butt VS, Davies DR, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radical Res 23:517–532
Bradley PM, Chapelle FH, Lovley DR (1998) Humic acids as electron acceptors for anaerobic microbial oxidation of vinyl chloride and dichloroethene. Appl Environ Microbiol 64:3102–3105
Brady NC, Weil RR (2010) Elements of the nature and properties of soils. Pearson Education International, New Jersey
Bressy P (1996) La bio-électronique et les mystères de la vie. Cours élémentaire pratique et théorie d’initiation à la bio-électronique selon la méthode Louis-Claude Vincent. Le courrier du livre, Paris
Brzezinska M (2004) Aeration status of soil and enzyme activity. In: Glinski J, Jozefaciuk G, Stahr K (eds) Soil-plant-atmosphere aeration and environmental problems. Agrophysics/Hohenheim University/Institure of Agrophysics Polish Academy of Science, Lubli-Stuttgart, pp 55–59
Carter CE (1980) Redox potential and sugarcane yield relationship. T ASABE 23:0924–0927
Chaboussou F (1985) Santé des cultures: une révolution agronomique. La maison rustique, Flammarion
Chadwick OA, Chorover J (2001) The chemistry of pedogenic thresholds. Geoderma 100:321–353
Chaignon V, Bedin F, Hinsinger P (2002) Copper bioavailability and rhizosphere pH changes as affected by nitrogen supply for tomato and oilseed rape cropped on an acidic and a calcareous soil. Plant Soil 243:219–228
Chang FC, Swenson RP (1997) Regulation of oxidation-reduction potentials through redox-linked ionization in the Y98H mutant of the Desulfovibrio vulgaris [Hildenborough] flavodoxin: direct proton nuclear magnetic resonance spectroscopic evidence for the redox-dependent shift in the pK(a) of histidine-98. Biochemistry 36:9013–9021
Charyulu PBBN, Rao VR (1980) Influence of various soil factors on nitrogen fixation by Azospirillum spp. Soil Biol Biochem 12:343–346
Chen J, Xuan J, Du C, Xie J (1997) Effect of potassium nutrition of rice on rhizosphere redox status. Plant Soil 188:131–137
Chesworth W (2004) Redox, soil and carbon sequestration. Edafologia 11:37–43
Chesworth W, Macias F (2004) Proton pumping, electron pumping, and the activities of aluminosilicate components in the formation of Andosols. In: Oskarsson H, Arnalds O (eds) Volcanic soil resources in Europe Cost Action 622 final meeting. RALA/ Agricultural Research Institute, Reyjavik
Chesworth W, Cortizas AM, García-Rodeja E (2006) The redox-pH approach to the geochemistry of the Earth's land surface, with application to peatlands. In: Martini IP, Martinez-Cortizas A, Chesworth W (eds) Developments in Earth surface processes, vol 9. Elsevier, Amsterdam, pp 175–195
Clark WM (1960) Oxidation-reduction potentials of organic systems. Williams and Wilkins, Baltimore
Clay DE, Clapp CE, Linden DR, Molina JAE (1992) Tillage influence on redox potentials following rainfall. Soil Till Res 22:211–219
Cocheme HM, Murphy MR (2009) The uptake and interactions of the redox cycler paraquat with mitochondria. Method Enzymol 456:395–417
Cornelissen JHC, Sibma F, Van Logtestijn RSP, Broekman RA, Thompson K (2011) Leaf pH as a plant trait: species-driven rather than soil-driven variation. Funct Ecol 25:449–455
Czyz EA (2004) Effects of traffic on soil aeration, bulk density and growth of spring barley. Soil Till Res 79:153–166
Davet P (1996) Vie microbienne du sol et production végétale. INRA, Paris
De Gara L, Locato V, Dipierro S, de Pinto MC (2010) Redox homeostasis in plants. The challenge of living with endogenous oxygen production. Resp Physiol Neuro 173:S13–S19
De Mars H, Wassen MJ (1999) Redox potentials in relation to water levels in different mire types in the Netherlands and Poland. Plant Ecol 140:41–51
De Orellana JA, Pilatti MA (1999) The ideal soil: I. An edaphic paradigm for sustainable agriculture. J Sustainable Agric 15:47–59
DeAngelis KM, Silver WL, Thompson AW, Firestone MK (2010) Microbial communities acclimate to recurring changes in soil redox potential status. Environ Microbiol 12:3137–3149
DeLaune RD, Devai I, Mulbah C, Crozier C, Lindau CW (1997) The influence of soil redox conditions on atrazine degradation in wetlands. Agr Ecosys Env 66:41–46
Dent DL (1993) Bottom-up and top-down development of acid sulphate soils. Catena 20:419–425
Deribere M (1949) Les applications industrielles du rH et le potentiel d'oxydo-réduction. Dunod, Paris
Dessaux Y, Hinsinger P, Lemanceau P (2009) Rhizosphere: so many achievements and even more challenges. Plant Soil 321:1–3
Dietz KJ (2003) Redox control, redox signaling, and redox homeostasis in plant cells. Int Rev Cytol 228:141–193
Dietz KJ, Pfannschmidt T (2011) Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol 155:1477–1485
Dietz KJ, Scheibe R (2004) Redox regulation: an introduction. Physiol Plant 120:1–3
Dietzel KA, Ketterings QM, Rao R (2009) Predictors of lime needs for ph and aluminum management of new york agricultural soils. Soil Sci Soc Am J 73:443–448
Ding H, Hidalgo E, Demple B (1996) The redox state of the [2Fe-2S] clusters in SoxR protein regulates its activity as a transcription factor. J Biol Chem 271:33173–33175
Dore T, Makowski D, Malezieux E, Munier-Jolain N, Tchamitchian M, Tittonell P (2011) Facing up to the paradigm of ecological intensification in agronomy: revisiting methods, concepts and knowledge. Eur J Agron 34:197–210
Downum KR (1986) Photoactivated biocides from higher plants. In: Green MB, Hedin PA (eds) Natural resistance to pests roles of allelochemicals. ACS, Washington, pp 197–205
Downum KR, Rodriguez E (1986) Toxicological action and ecological importance of plant photosensitizers. J Chem Ecol 12:823–834
Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, De Paepe R (2003) Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. Plant Cell 15:1212–1226
Dwire KA, Kauffman JB, Baham JE (2006) Plant species distribution in relation to water-table depth and soil redox potential in Montane riparian meadows. Wetlands 26:131–146
Eagleson M (1993) Concise encyclopedia chemistry (ABC Chimie). De Gruyter, Berlin
Evans DE (2004) Aerenchyma formation. New Phytol 161:35–49
Falkowski PG, Fenchel T, Delong EF (2008) The microbial engines that drive Earth's biogeochemical cycles. Science 320:1034–1039
Felle H (1988) Short-term pH regulation in plants. Physiol Plant 74:583–591
Fenchel T, King GM, Blackburn TH (1998) Bacterial biogeochemistry. The ecophysiology of mineral cycling. Academic, San Diego
Fernandez MR, Zentner RP, Basnyat P, Gehl D, Selles F, Huber D (2009) Glyphosate associations with cereal diseases caused by Fusarium spp. in the Canadian Prairies. Eur J Agron 31:133–143
Fiedler S, Vepraskas MJ, Richardson JL (2007) Soil redox potential: Importance, field measurements, and observations. Adv Agron 94:1–54
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Filip Z (1973) Clay minerals as a factor influencing the biochemical activity of soil microorganisms. Folia Microbiol 18:56–74
Fischer W, Flessa H, Schaller G (1989) pH values and redox potentials in microsites of the rhizosphere. J Plant Nutr Soil Sci 152:191–195
Flessa H, Fischer W (1992a) Plant-induced changes in the redox potentials of rice rhizospheres. Plant Soil 143:55–60
Flessa H, Fischer W (1992b) Redox processes in the rhizosphere of terrestrial and paludal plants. J Plant Nutr Soil Sci 155:373–378
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Fougerousse A (1996) Le potentiel redox E et le rH2: deux approches de l’évaluation de la force des oxydants et des réducteurs. B Union Phys 90:319–331
Foyer CH, Noctor G (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant cell 17:1866–1875
Frohne T, Rinklebe J, Diaz-Bone RA, Du Laing G (2011) Controlled variation of redox conditions in a floodplain soil: Impact on metal mobilization and biomethylation of arsenic and antimony. Geoderma 160:414–424
Gambrell RP, Patrick WH Jr (1978) Chemical and microbiological properties of anaerobic soils and sediments. In: Hook DD, Crawford RM (eds) Plant life in anaerobic environments. Ann Arbor Science, Ann Arbor, pp 375–423
Gantimurov LL (1969) Studies in theoretical and applied soil science. Nauka Publ, Novosibirsk
Garrett SD (1963) Soil fungi and soil fertility. Pergamon, Oxford
Gerendas J, Schurr U (1999) Physicochemical aspects of ion relations and pH regulation inplants—a quantitative approach. J Exp Bot 50:1101–1114
Ghezzi P (2005) Regulation of protein function by glutathionylation. Free Radical Res 39:573–580
Gilbert B, Frenzel P (1998) Rice roots and CH4 oxidation: the activity of bacteria, their distribution and the microenvironment. Soil Biol Biochem 30:1903–1916
Glinski J, Stepniewski W (1985) Soil aeration and its role for plants. CRC, Boca Raton
Gobat J-M, Aragno M, Matthey W (1998) Le sol vivant. Bases de pédologie. Biologie des sols. Presses polytechniques et universitaires romandes, Lausanne
Gotoh Y, Tai K (1957) On the differences of oxidizing power of paddy rice seedling roots among some varieties. Soil Sci Plant Nutr 2:198–200
Gotoh S, Yamashita K (1966) Oxidation-reduction potential of a paddy soil in situ with special reference to the production of ferrous iron, manganous manganese and sulfide. Soil Sci Plant Nutr 12:24–32
Govindjee, Krogmann D (2004) Discoveries in oxygenic photosynthesis (1727–2003): a perspective. Photosynth Res 80:15–57
Grable AR, Siemer EG (1967) Effects of bulk density, aggregate size, and soil water suction on oxygen diffusion, redox potentials, and elongation of corn roots. Soil Sci Soc Am J 32:180–186
Greenberg RC (1998) Understanding the redox (rH2) measurement of the biological terrain: Part I. Biomed Therap 16:156–158
Greenland DJ, Hayes MHB (1981) The chemistry of soil processes. Wiley, New York
Guan H, Schulze-Makuch D, Schaffer S, Pillai SD (2003) The effect of critical pH on virus fate and transport in saturated porous medium. Ground Water 41:701–708
Guérin B (2004) Bioénergétique. EDP, Paris
Hackett FE, Strettan JS (1928) The capillary pull of an ideal soil. J Agr Sci 18:671–681
Hanke GT, Holtgrefe S, Konig N, Strodtkotter I, Voss I, Scheibe R (2009) Use of transgenic plants to uncover strategies for maintenance of redox homeostasis during photosynthesis. Adv Bot Res 52:207-+
Hansen JM, Go YM, Jones DP (2006) Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol 46:215–234
Happonen LJ, Redder P, Peng X, Reigstad LJ, Prangishvili D, Butcher SJ (2010) Familial relationships in hyperthermo- and acidophilic Archaeal viruses. J Virol 84:4747–4754
Hartmann A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Heijnen CE, van Veen JA (1991) A determination of protective microhabitats for bacteria introduced into soil. FEMS Microbiol Lett 85:73–80
Heintze SG (1934) The use of the glass electrode in soil reaction and oxidation-reduction potential measurements. J Agric Sci 1934(24):28–41
Helrich CS, Budiardja RD, Sprunger PH (2001) The effect of pH on the conductivity of the ion channel in Southern Cowpea Mosaic Virus. Biophys J 80:1038
Herbel MJ, Suarez DL, Goldberg S, Gao S (2007) Evaluation of chemical amendments for pH and redox stabilization in aqueous suspensions of three California soils. Soil Sci Soc Am J 71:927–939
Heron G, Christensen TH, Tjell JC (1994) Oxidation capacity of aquifer sediments. Environ Sci Technol 28:153–158
Hill S (1988) How is nitrogenase regulated by oxygen? FEMS Microbiol Lett 54:111–129
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152
Höper H, Steinberg C, Alabouvette C (1995) Involvement of clay type and pH in the mechanisms of soil suppressiveness to fusarium wilt of flax. Soil Biol Biochem 27:955–967
Horn R, Peth S (2009) Soil structure formation and management effects on gas emission. Biologia 64:449–453
Huang PM, Germida JJ (2002) Chemical and biological processes in the rhizosphere: metal pollutants. In: Huang PM, Bollag J-M, Senesi N (eds) Interactions between soil particles and microorganisms impact on the terrestrial ecosystem. Wiley, Chichester, pp 381–438
Huner NPA, Maxwell DP, Gray GR, Savitch LV, Krol M, Ivanov AG, Falk S (1996) Sensing environmental temperature change through imbalances between energy supply and energy consumption: redox state of photosystem II. Physiol Plant 98:358–364
Husson O, Phung MT, Van Mensvoort MEF (2000a) Soil and water indicators for optimal practices when reclaiming acid sulphate soils in the Plain of Reeds, Viet Nam. Agric Water Manage 45:127–143
Husson O, Verburg PH, Phung MT, Van Mensvoort MEF (2000b) Spatial variability of acid sulphate soils in the Plain of Reeds, Mekong delta, Vietnam. Geoderma 97:1–19
Husson O, Phung MT, Fresco LO (2001) The effects of micro-elevation, soil, and water on rice growth and yield in acid sulphate soils in the Plain of Reeds, Vietnam. Trop Agric 78:29–36
Huybrechts M (1939) Le pH et sa mesure, les potentiels d’oxydo-réduction, le rH. Masson, Paris
Janssen BH, de Willigen P (2006) Ideal and saturated soil fertility as bench marks in nutrient management: 1. Outline of the framework. Agric Ecosys Environ 116:132–146
Jones DL, Hodge A, Kuzuakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Kandlbinder A, Wormuth D, Baier M, Scheibe R, Dietz KJ (2003) Redox control of chloroplast and nuclear gene expression. Free Radical Res 37:24–24
Kandlbinder A, Finkemeier I, Wormuth D, Hanitzsch M, Dietz KJ (2004) The antioxidant status of photosynthesizing leaves under nutrient deficiency: redox regulation, gene expression and antioxidant activity in Arabidopsis thaliana. Physiol Plant 120:63–73
Kashem MA, Singh BR (2001) Metal availability in contaminated soils: I. Effects of flooding and organic matter on changes in Eh, pH and solubility of Cd, Ni and Zn. Nutr Cycl Agroecosys 61:247–255
Kaurichev IS, Orlov DS (1982) Redox processes and their role in the genesis and fertility of soils. Kolos, Moscow
Kaurichev IS and Shishova VS (1967) Oxidation reduction conditions of coarse textured soils of the Meschera lowland. Sov Soil Sci+ 5:636–646
Kaurichev IS, Tararina LF (1972) On redox conditions inside and outside of aggregates in a grey forest soil. Pochvovedenie 10:39–42
Keen BA (1924) On the moisture relationships in an ideal soil. J Agric Sci 14:170–177
Kemmou S, Dafir JE, Wartiti M, Taoufik M (2006) Seasonal variations and potential mobility of sediment phosphorus in the Al Massira reservoir, Morocco. Water Qual Res J Can 41:427–436
Kimbrough DE, Kouame Y, Moheban P, Springthorpe S (2006) The effect of electrolysis and oxidation-reduction potential on microbial survival, growth and disinfection. Int J Environ Pollut 27:211–221
Kludze HK, DeLaune RD (1999) Gaseous exchange and wetland plant response to soil redox intensity and capacity. Soil Sci Soc Am J 59:939–945
Kogel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kolbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Kopittke PM, Menzies NW (2007) A review of the use of the basic cation saturation ratio and the 'Ideal' soil. Soil Sci Soc Am J 71:259–265
Kovda VA (1973) The basics of studying soils. Nauka Publ, Moscow
Kralova M, Masscheleyn PH, Patrick WH Jr (1992) Redox potential as an indicator of electron availability for microbial activity and nitrogen transformations in aerobic soil. Z Mikrobiol 147:388–399
Krumbein WC, Garrels RM (1952) Origin and classification of chemical sediments in term of pH and oxidation-reduction potentials. J Geol 60:1–15
Kurkdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Biol 40:271–303
Kuzniak E (2010) The ascorbate-glutathione cycle and related redox signals in plant-pathogen interactions. In: Anjum NA, Umar S, Chan MT (eds) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht, pp 115–136
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. Review. J Plant Nutr Soil Sc 163:421–431
Kyle JE, Pedersen K, Ferris FG (2008) Virus Mineralization at Low pH in the Rio Tinto, Spain. Geomicrobiol J 25:338–345
Laanbroek HJ (1990) Bacterial cycling of minerals that affect plant growth in waterlogged soils: a review. Aquat Bot 38:109–125
Ladha JK, Dawe D, Pathak H, Padre AT, Yadav RL, Singh B, Singh Y, Singh Y, Singh P, Kundu AL, Sakal R, Ram N, Regmi AP, Gami SK, Bhandari AL, Amin R, Yadav CR, Bhattarai EM, Das S, Aggarwal HP, Gupta RK, Hobbs PR (2003) How extensive are yield declines in long-term rice-wheat experiments in Asia? Field Crop Res 81:159–180
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Biol 48:251–275
Lambers H, Chapin SFI, Pons TL (2008) Plant physiological ecology. Springer, New York
Lambers H, Mougel C, Jaillard B, Hinsinger P (2009) Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil 321:83–115
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Lefroy RDB, Samosir SSR, Blair GJ (1993) the dynamics of sulfur, phosphorus and iron in flooded soils as affected by changes in Eh and Ph. Aust J Soil Res 31:493–508
Li C (2007) Quantifying greenhouse gas emissions from soils: scientific basis and modeling approach. Soil Sci Plant Nutr 53:344–352
Lindroth RL, Hemming JDC (1990) Responses of the gypsy moth (Lepidoptera: Lymantriidae) to tremulacin, an aspen phenolic glycoside. Environ Entomol 19:842–847
Lindsay WL (1979) Chemical equilibria in soils. Wiley-Interscience, New York
Lindsay WL (1983) Use of pe + pH to predict and interpret metal solubility relationships in soils. Sci Total Environ 28:169–178
Lovley DR, Fraga JL, B-H EL, Hayes LA, Phillips EJP, Coates JD (1998) Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol 26:152–157
Luthje S, Doring O, Heuer S, Luthen H, Bottger M (1997) Oxidoreductases in plant plasma membranes. Biochem Biophys Acta 1331:81–102
Macías F, Camps Arbestain M (2010) Soil carbon sequestration in a changing global environment. Mitig Adapt Strateg Glob Change 15:511–529
Magdoff FR, Bartlett RJ (1984) Soil pH buffering revisited. Soil Sci Soc Am J 49:145–148
Manahan SF (2001) Fundamentals of environmental chemistry. Second edition. CRC Press
Mansfeldt T (2003) In situ long-term redox potential measurements in a dyked marsh soil. J Plant Nutr Soil Sci 166:210–219
Marino D, Pucciariello C, Puppo A, Frendo P, Jacquot J-P (2009) The redox state, a referee of the legume-rhizobia symbiotic game. Adv Bot Res 52:115–151
Marschner H (1991) Mechanisms of adaptation of plants to acid soils. Plant Soil 134:1–20
Marschner P (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Masaphy S, Henis Y, Levanon D (1996) Manganese-enhanced biotransformation of atrazine by the white rot fungus Pleurotus pulmonarius and its correlation with oxidation activity. Appl Environ Microbiol 62:3587–3593
Masscheleyn PH, DeLaune RD, Patrick WH Jr (1993) Methane and nitrous oxide emissions from laboratory measurements of rice soil suspension: effect of soil oxidation-reduction status. Chemosphere 26:251–260
Mateo A, Funck D, Mahlenbock P, Kular B, Mullineaux PM, Karpinski S (2006) Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J Exp Bot 57:1795–1807
Mathis P (1995) Photosynthesis: from Light to Biosphere In Proceedings of the Xth International Photosynthesis Congress Volume III, Montpellier, 20–25 August 1995, France
Meadows PS, Reichelt AC, Meadows A, Waterworth JS (1994) Microbial and meiofaunal abundance, redox potential, pH and shear strength profiles in deep sea Pacific sediments. J Geol Soc 151:377–390
Meyer GA, Montgomery ME (1987) Relationships between leaf age and the food quality of cottonwood foliage for the gypsy moth, Lymantria dispar. Oecologia 72:527–532
Miller AL, Smith GN, Raven JA, Gow NAR (1991a) Ion currents and the nitrogen status of roots of Hordeum vulgare and nonnodulated Trifolium repens. Plant Cell Environ 14:559–567
Miller FC, Macauley BJ, Haeper ER (1991b) Investigation of various gases, pH and redox potential in mushroom composting Phase I stacks. Aust J Exp Agr 1991:415–425
Minuto A, Gaggero L, Gullino ML, Garibaldi A (2008) Influence of pH, nutrient solution disinfestation and antagonists application in a closed soilless system on severity of Fusarium wilt of gerbera. Phytoparasitica 36:294–303
Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135:702–708
Mullineaux P, Rausch T (2005) Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth Res 86:459–474
Neumann G, Römheld V (2000) The release of root exudates as affected by the plant physiological status. In: Pinton R, Varanini Z, Nannipieri Z (eds) The rhizosphere: biochemistry and organic substances at the soil-plant interface. Dekker, New York, pp 23–72
Noctor G (2006) Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ 29:409–425
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Phys 49:249–279
Noctor G, Veljovic-Jovanovic S, Foyer CH (2000) Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philos T Roy Soc B 355:1465
Oglesby J P (1997) Redox buffering by soil organic matter. Abst Pap Am Chem S 213:147-GEOC
Olness A, Rinke J, Hung H-M, Evans SD (1989) Effect of tillage on redox potential of a Tara Silt Loam soil. Soil Sci 148(4):265–274
Orszagh J (1992) Quelques aspects physico-chimiques des coordonnées bio-électroniques. Sciences du vivant 4:45–62
Osuna-Canizalez F, De Datta S, Bonman J (1991) Nitrogen form and silicon nutrition effects on resistance to blast disease of rice. Plant Soil 135:223–231
Owabor CN, Obahiagbon KO (2009) Assessing the rate of remediation of polycyclic aromatic hydrocarbon contaminated soil using redox parameters. In: Ibhadode AOA, Igbafe IA, Anyata BU (eds) Advances in materials and systems technologies II. Trans Tech Publications Ltd, Stafa-Zurich, pp 439–444
Paillet Y, Cassagne N, Brun JJ (2010) Monitoring forest soil properties with electrical resistivity. Biol Fertil Soils 46:451–460
Pasternak TP, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen HA, Dudits D, Fehar A (2002) The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol 129:1807–1819
Patsoukis N, Georgiou CD (2007a) Effect of glutathione biosynthesis-related modulators on the thiol redox state enzymes and on sclerotial differentiation of filamentous phytopathogenic fungi. Mycopathologia 163:335–347
Patsoukis N, Georgiou CD (2007b) Effect of sulfite-hydrosulfite and nitrite on thiol redox state, oxidative stress and sclerotial differentiation of filamentous phytopathogenic fungi. Pestic Biochem Physiol 88:226–235
Patsoukis N, Georgiou CD (2007c) Effect of thiol redox state modulators on oxidative stress and sclerotial differentiation of the phytopathogenic fungus Rhizoctonia solani. Arch Microbiol 188:225–233
Paustian K, Andrén O, Janzen HH, Lal R, Smith P, Tian G, Tiessen H, Van Noordwijk M, Woomer PL (1997) Agricultural soils as a sink to mitigate CO2 emissions. Soil Use Manage 13:230–244
Payne PA, Asher MJC, Kershaw CD (1994) The incidence of pythium Spp and aphanomyces cochlioides associated with the sugar-beet growing soils of Britain. Plant Pathol 43:300–308
Pearsall WH, Mortimer CH (1939) Oxidation-reduction potentials in waterlogged soils, natural waters and muds. J Ecol 27:483–501
Pekosz A, Gonzalez-Scarano F (1996) The extracellular domain of La Crosse virus G1 forms oligomers and undergoes pH-dependent conformational changes. Virology 225:243–247
Pennington MR, Walters MB (2006) The response of planted trees to vegetation zonation and soil, redox potential in created wetlands. For Ecol Manage 233:1–10
Pett-Ridge J, Firestone MK (2005) Redox fluctuation structures microbial communities in a wet tropical soil. Appl Environ Microbiol 71:6998–7007
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312
Pfannschmidt T (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 8:33–41
Phillips IR (1998) Phosphorus availability and sorption under alternating waterlogged and drying conditions. Commun Soil Sci Plant 29:3045–3059
Pilatti MA, de Orellana JA (2000) The ideal soil: II. Critical values of an 'ideal soil' for Mollisols in the North of the Pampean Region (in Argentina). J Sustain Agr 17:89–112
Ponnamperuma FN (1965) Dynamic aspects of flooded soils and the nutrition of the rice plant. In IRRI Symposium on Mineral Nutrition of the Rice Plant. Johns Hopkins Press, Baltimore, pp 295–328
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc Roy Soc Lond B 84:260–278
Potters G, Horemans N, Jansen MAK (2010) The cellular redox state in plant stress biology—a charging concept. Plant Physiol Bioch 48:292–300
Pourbaix MJN (1945) Thermodynamique des solutions aqueuses diluées. Représentation graphique du rôle du pH et du Potentiel. PhD Thesis Delft, p 142
Puigdomenech I (2009–2011) Medusa software https://sites.google.com/site/chemdiagr/ accessed last on 2012/08/23
Rabenhorst AC, Hively WD, James BR (2009) Measurements of soil redox potential. Soil Sci Soc Am J 73:668–674
Rabotnova IL, Schwartz W (1962) The importance of physical-chemical factors (pH and rH2) for the life activity of microorganisms. VEB Gustav Fischer Verlag, Berlin
Raven JA (1986) Biochemical disposal of excess H + in growing plants? New Phytol 104:175–206
Raven JA, Smith FA (1976) Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytol 76:415–431
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC, Boca Raton
Reddy KR, Feijtel TC, Patrick WHJ (1986) Effect of soil redox condition on microbial oxidation of organic matter. In: Chen Y, Avnimelech Y (eds) The role of organic matter in modern agriculture. Kluwer Academic Publishers, Dordrecht, pp 117–160
Rengel Z (2002) Handbook of plant growth. pH as the master variable. Dekker, New York
Richard G, Besson A, Sani AA, Cosenza P, Boizard H, Cousin I (2006) A new approach of soil structure characterisation in field conditions based on electrical resistivity measurements. In: Horn R, Fleige H, Peth S, Peng XH (eds) Soil management for sustainability. Catena Verlag, Reiskirchen, pp 415–421
Rigalma K, Duvaux-Ponter C, Gallouin F, Roussel S (2009) Les courants électriques parasites en élevage. Inra Prod Anim 22:291–302
Rittmann BE (2006) Microbial ecology to manage processes in environmental biotechnology. Trends Biotechnol 24:261–266
Roger P, Garcia J-L (2001) Introduction à la microbiologie du sol. Université de Provence, Ecole supérieure d’ingénieures de Luminy, France
Rossiter MC, Schultz JC, Baldwin IT (1988) Relationships among defoliation, red oak phenolics, and gypsy moth growth and reproduction. Ecology 69:267–277
Rosso D, Bode R, Li WZ, Krol M, Saccon D, Wang S, Schillaci LA, Rodermel SR, Maxwell DP, Huner NPA (2009) Photosynthetic redox imbalance governs leaf sectoring in the Arabidopsis thaliana variegation mutants immutans, spotty, var1, and var2. Plant Cell 21:3473–3492
Roth SK, Lindroth RL, Montgomery ME (1994) Effects of foliar phenolics and ascorbic acid on performance of the gypsy moth (Lymantria dispar). Biochem Syst Ecol 22:341–351
Roth S, Knorr C, Lindroth RL (1997) Dietary phenolics affects performance of the gypsy moth (Lepidoptera: Lymantriidae) and its parasitoid Cotesia melanoscela (Hymenoptera: Braconidae). Environ Entomol 26:668–671
Rousseau J (1959) Etude du sol. Rev Pharm Ouest Janvier 1959:22
Rusanov AM, Anilova LV (2009) The humus formation and humus in forest-steppe and steppe chernozems of the southern Cisural region. Eurasian Soil Sci+ 42:1101–1108
Sabiene N, Kusliene G, Zaleckas E (2010) The influence of land use on soil organic carbon and nitrogen content and redox potential. Zemdirbyste 97:15–24
Sakano K (1998) Revision of biochemical pH-stat: involvement of alternative pathway metabolisms. Plant Cell Physiol 39:467
Sallade YE, Sims JT (1997) Phosphorus transformations in the sediments of Delaware's agricultural drainageways: II. Effect of reducing conditions on phosphorus release. J Environ Qual 26:1579–1588
Sam T, Eecen G-J, Pley C, Bosch L, Mandel M (1991) Thermal stability of turnip yellow mosaic virus RNA: effect of pH and multivalent cations on RNA deaggregation and degradation. BBA Gene Struct Expr 1129:64–72
Samouëlian A, Cousin I, Tabbagh A, Bruand A, Richard G (2005) Electrical resistivity survey in soil science: a review. Soil Till Res 83:173–193
Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Leaver CJ, Van Montagu M, Inze D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. P Natl Acad Sci Biol 94:2745–2750
Sanon A, Andrianjaka ZN, Prin Y, Bally R, Thioulouse J, Comte G, Duponnois R (2009) Rhizosphere microbiota interfers with plant-plant interactions. Plant Soil 321:259–278
Saurina J, Hernandez-Cassou S, Fabregas E, Alegret S (2000) Cyclic voltammetric simultaneous determination of oxidizable amino acids using multivariate calibration methods. Anal Chim Acta 405:153–160
Savant NK, Ellis RJ (1964) Changes in redox potential and phosphorus availability in submerged soil. Soil Sci 98:388–394
Savich VI, Kaurichev IS, Draman K (1980) Amendments for regulating the oxidation-reduction potential of soils. Izvestiya Timiryazevskoi Sel'skokhozyaistvennoi Akademii 1980:75–82
Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56:1481–1489
Schwab AP, Lindsay WL (1982) The effect of redox on the solubility and availability of manganese in a calcareous soil. Soil Sci Soc Am J 47:217–220
Scrivanti LR, Zunino MP, Zygadlo JA (2003) Tagetes minuta and Schinus areira essential oils as allelopathic agents. Biochem Syst Ecol 31:563–572
Seladji S, Cosenza P, Tabbagh A, Ranger J, Richard G (2010) The effect of compaction on soil electrical resistivity: a laboratory investigation. Eur J Soil Sci 61:1043–1055
Seo DC, DeLaune RD (2010) Effect of redox conditions on bacterial and fungal biomass and carbon dioxide production in Louisiana coastal swamp forest sediment. Sci Total Environ 408:3623–3631
Shinmura A (2004) Principle and effect of soil sterilization methods by reducing the redox potential of soil. In PSJ Soilborne Disease Workshop Report 22. pp 2–12
Shlomai J (2010) Redox control of protein-DNA interactions: from molecular mechanisms to significance in signal transduction, gene expression, and DNA replication. Antioxid Redox Signal 13:1429–1476
Sillen LG, Martell AE (1964) Stability constants of metal-ion complexes. Chem Soc London Spec 17:1–38
Simek M, Cooper JE (2002) The influence of soil pH on denitrification: progress towards the understanding of this interaction over the last 50 years. Eur J Soil Sci 53:345–354
Skyllberg U, Raulund-Rasmussen K, Borggaard OK (2001) pH buffering in acidic soils developed under Picea abies and Quercus robur—effects of soil organic matter, adsorbed cations and soil solution ionic strength. Biogeochemistry 56:51–74
Smith FA, Raven JA (1979) Intracellular pH and its regulation. Annu Rev Plant Phys 30:289–311
Snakin VV, Dubinin AG (1980) Use of the oxidation potential of soils for the thermodynamic characterisation of the biogeocenotic processes. Doklady Akademii Nauk SSSR 252:464–466
Snakin VV, Prisyazhnaya AA, Kovacs-Lang E (2001) Soil liquid phase composition. Elsevier Science B.V, Amsterdam
Son Y, Oh M, Lee S (2010) Estimation of soil weathering degree using electrical resistivity. Environ Earth Sci 59:1319–1326
Sparks DL (2001) Elucidating the fundamental chemistry of soils: past and recent achievements and future frontiers. Geoderma 100:303–319
Sposito G (1989) The chemistry of soils. Oxford University press, New York
Stepniewska Z, Wolinska A, Klin R (2009) Heavy metals release from eroded loess soils. Int Agrophysics 23:377–382
Stepniewski W, Stepniewska Z (2009) Selected oxygen-dependent process-response to soil management and tillage. Soil Till Res 102:193–200
Steyaert JM, Weld RJ, Stewart A (2010) Ambient pH intrinsically influences Trichoderma conidiation and colony morphology. Fungal Biol 114:198–208
Stolzy LH, Letey J (1964) Characterizing soil oxygen conditions with a platinum microelectrode. Adv Agron 16:249–279
Stoop WA, Uphoff N, Kassam A (2002) A review of agricultural research issues raised by the system of rice intensification (SRI) from Madagascar: opportunities for improving farming systems for resource-poor farmers. Agr Syst 71:249–274
Stout M (1949) Relation of oxidation-reduction potentiel, respiration, and catalase activity in induction of reproductive development in sugar beets. Bot Gaz 10
Stumm W (1966) Redox potential as an environmental parameter; conceptual significance and operational limitation. In 3rd Internat Conf on Water Pollution Research. pp 1–16, Munich
Stumm W, Morgan JJ (1981) Aquatic chemistry. An introduction emphasizing chemical equilibria in natural waters. Wiley, New York
Sweetlove LJ, Møller IM, Jacquot J-P (2009) Oxidation of proteins in plants—mechanisms and consequences. Adv Bot Res 52:1–23
Szent-Gyorgyi A (1957) Bioenergetics. Academic, New York
Szent-Gyorgyi A (1960) Introduction to submolecular biology. Academic, New York
Taiz L (1992) The plant vacuole. J Exp Biol 172:113–122
Takehara T, Hanzawa S, Funabara M, Nakaho K, Nakagawa A (2004) Control of soilborne pathogens using allelopathic plants to lower redox potential of soil. Phytopathology 94:S101–S101
Tanaka A, Loe R, Navasero SA (1966) Some mechanisms involved in the development of iron toxicity symptoms in the rice plant. Soil Sci Plant Nutr 12:32–37
Theng BKG, Orchard VA (1995) Interactions of clays with microorganisms and bacterial survival in soil: a physicochemical perspective. In: Huang PM, Berthelin J, Bollag J-M, McGill WB, Page AL (eds) Environmental impact of soil component interactions Vol 2 metals, other inorganics, and microbial activities. CRC Press/Lewis, Boca Raton, pp 123–143
Tittonell P, Vanlauwe B, Corbeels M, Giller KE (2008) Yield gaps, nutrient use efficiencies and response to fertilisers by maize across heterogeneous smallholder farms of western Kenya. Plant Soil 313:19–37
Tomilov A, Tomilova N, Shin DH, Jamison D, Torres M, Reagan R, Horning T, Truong R, Nava AJ, Nava A, Yoder JI (2006) Chemical signaling between plants: mechanistic similarities between phytotoxic allelopathy and host recognition by parasitic plants. In: Dicke M, Takken W (eds) Chemical ecology: from gene to ecosystem. Springer, Wageningen, pp 55–69
Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425:393–397
Truesdell A (1969) The advantages of using pe rather than Eh in redox equilibrium calculation. J Geol Ed 17:17–20
Turpaev KT, Litvinov D (2004) Redox-dependent regulation of gene expression induced by nitric oxide. Mol Biol (Mosk) 38:56–68
Twining JR, Zaw M, Russell R, Wilde K (2004) Seasonal changes of redox potential and microbial activity in two agricultural soils of tropical Australia: some implications for soil-to-plant transfer of radionuclides. J Environ Radioact 76:265–272
Ugwuegbu BU, Prasher SO, Ahmad D, Dutilleul P (2001) Bioremediation of residual fertilizer nitrate: II. Soil redox potential and soluble iron as indicators of soil health during treatment. J Environ Qual 30:11–18
Unger IM, Muzika RM, Motavalli PP, Kabrick J (2008) Evaluation of continuous in situ monitoring of soil changes with varying flooding regimes. Commun Soil Sci Plant 39:1600–1619
Vadas PA, Sims JT (1998) Redox status, poultry litter, and phosphorus solubility in Atlantic Coastal plain soils. Soil Sci Soc Am J 62:1025–1034
Van Breemen N (1987) Effect of redox processes on soil acidity. Neth J Agr Sci 35:271–279
Vincent L-C (1956) Potentiel d’oxydo-réduction et rH2. Bioélectronique et médecine. Rev Pathol Gen Physio Clin 1956:42
Vlès F (1927) Cours de physique biologique. Tome premier. Introduction à la chimie-physique biologique. Vigot Frères, Paris
Vlès F (1929) Précis de chimie-physique à l’usage des étudiants en médecine. Vigot Frères, Paris
Voisin A (1959) Sol, herbe, cancer. La santé de l'animal et de l'homme dépend de l'équilibre du sol. La Maison Rustique, Paris
Volk NJ (1993) The effect of oxidation–reduction potential on plant growth. J Am Soc Agron 31:665–670
Von de Kammer F, Thöming J, Förstner U (2000) Redox buffer capacity concept as a tool for the assessment of long-term effects in natural attenuation / intrinsic remediation. In: Schüring J, Schultz HD, Fischer WR, Böttcher J, Duijnisveld WHM (eds) Redox: fundamentals, processes and applications. Springer-Verlag, Berlin, pp 189–202
Vortex (2008–2011) http://idee-aquaculture.fr/competences/assistance-technique# accessed last on 2012/08/23
Wang ZP, Delaune RD, Masscheleyn PH, Patrick WH (1993) Soil redox and ph effects on methane production in a flooded rice soil. Soil Sci Soc Am J 57:382–385
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Whalen JK, Chang C, Clayton GW, Carefoot JP (2000) Cattle manure amendments can increase the pH of acid soils. Soil Sci Soc Am J 64:962–966
Whitfield AE, Ullman DE, German TL (2005) Tomato spotted wilt virus glycoprotein G(C) is cleaved at acidic pH. Virus Res 110:183–186
Wilson KE, Ivanov AG, Oquist G, Grodzinski B, Sarhan F, Huner NPA (2006) Energy balance, organellar redox status, and acclimation to environmental stress. Can J Bot/Rev Can Bot 84:1355–1370
Wlodarczyk T, Steniewska Z, Brzezinska M (2003) Denitrification, organic matter and redox potential transformations in Cambisols. Internat agrophysics 17:219–227
Wurmser R (1921) Recherches sur l’assimilation chlorophyllienne. Archiv Phys Biol 1921:33
Xing S, Lauri A, Zachgo S (2006) Redox regulation and flower development: a novel function for glutaredoxins. Plant Biol 8:547–555
Yadav SK (2010) Cold stress tolerance mechanisms in plants. A review. Agron Sustain Dev 30:515–527
Yamafuji K, Cho T (1948) Weitere Studien zur Entstehung des Seidenraupenpolyedervirus ohne Virusinfektion. Biochemische Z 318:95
Yamafuji K, Fujiki T (1947) Experimentelle Erzeugung des Tabakmosaikvirus. Biochemische Z 318:101–106
Yang J, Hu YM, Bu RC (2006) Microscale spatial variability of redox potential in surface soil. Soil Sci 171:747–753
Yoder JI (2001) Host-plant recognition by parasitic Scrophulariaceae. Curr Opin Plant Biol 4:359–365
Yoshida S (1981) Fundamentals of rice crop science. Intl. Rice Res. Inst, Los Banos
Youssef RA, Chino M (1989) Root-induced changes in the rhizosphere of plants. I. pH changes in relation to the bulk soil. Soil Sci Plant Nutr 35:461–468
Yu K, Patrick WH (2003) Redox range with minimum nitrous oxide and methane production in a rice soil under different pH. Soil Sci Soc Am J 67:1952–1958
Zakharievsky MS (1967) Oksredmetria (Redoximetry). Khimiya (Chimia) 1967:108–112
Acknowledgements
I would like to thank Prof. Alain Capillon, Prof. Norman Uphoff, Fabrice Dreyfus and Cécile Fovet-Rabot for their support and the many comments that they made to improve the manuscript, and also my colleagues in the SIA research unit at CIRAD for enduring the many discussions on redox potential. Thanks also go to Peter Biggins for revising the English. Finally, I deeply thank the anonymous reviewers for their very constructive comments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362, 389–417 (2013). https://doi.org/10.1007/s11104-012-1429-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1429-7