Abstract
The study was designed to evaluate the long-term efficacy and safety of the 28-day prolonged-release Autogel formulation of the somatostatin analogue lanreotide (Lan-Autogel) in unselected patients with acromegaly. The study comprised four phases: washout; a double-blind comparison with placebo, at a single randomized dose (60, 90 or 120 mg) of Lan-Autogel; a single-blind, fixed-dose phase for four injections (placebo group was re-allocated to active treatment); and eight injections with doses tailored according to biochemical response. Serum samples were assessed for growth hormone (GH) and insulin-like growth factor-1 (IGF-1) levels, at weeks 4, 13, 14, 15, 16, 32 and 52. 108 patients were enrolled and 99 completed 52 weeks’ treatment. Four weeks after the first injection, serum GH levels decreased by >50% from baseline in 63% of patients receiving Lan-Autogel compared with 0% receiving placebo (P < 0.001). After four injections, 72% of patients had a >50% reduction in GH levels; 49% patients achieved GH levels ≤ 2.5 ng/ml; 54% had normalized IGF-1; and 38% achieved the combined criterion of GH level ≤ 2.5 ng/ml and normalized IGF-1. The corresponding proportions by week 52 were 82, 54, 59 and 43%, respectively. In patients not requiring dose escalation to 120 mg, 85% achieved biochemical control (combined criterion). Treatment was well tolerated by all patients. In conclusion, Lan-Autogel was effective in controlling GH and IGF-1 hypersecretion in patients with acromegaly and showed a rapid onset of action.
Similar content being viewed by others
Introduction
Acromegaly is a rare disease, with an average annual incidence of approximately 3 per million and a prevalence of approximately 60 per million. It is caused by an over-production of growth hormone (GH) from a GH-secreting pituitary adenoma. Acromegaly can be associated with a variety of clinical features, and is usually characterized by an insidious progression of dysmorphic skeletal and soft-tissue growth, severe sweating, headaches, arthritis and, in some patients, visual field loss [1]. Impaired cardiac and respiratory functions are the most serious features of the disease, and these result in morbidity and mortality more than double the rate of the general population [2–5]. Biochemical control of the disease is primarily achieved through surgical removal of the tumour. Radiotherapy can also be used in some patients with recurrent or persistent tumours [1]. In patients where these treatments are inappropriate, or have proven to be unsuccessful, somatostatin analogues are the recommended medical therapy [6, 7]. Indeed, treatment with somatostatin analogues is increasingly considered as a primary medical intervention in selected patients.
The somatostatin analogue lanreotide (Somatuline®, Ipsen) has demonstrated efficacy in the medical management of acromegaly [8–11]. Somatuline Autogel® (Lan-Autogel) (marketed as Somatuline Depot® in the US) is a prolonged-release, supersaturated formulation of lanreotide, which is available in sterile, ready-to-use syringes. Lan-Autogel has good efficacy in the management of acromegaly [12], including in long-term treatment [13–15], and has a similar efficacy and safety profile to octreotide [16, 17]. This somatostatin analogue formulation has also simplified treatment by increasing the dosing interval, potentially improving patient compliance and providing the possibility of patient self-administration [18]. After deep subcutaneous injection, the controlled release of lanreotide maintains therapeutic levels beyond the 28-day dosing period [19–21]. In this study (NCT number 00234572), the efficacy and safety of a single dose of Lan-Autogel was compared with placebo in a large international cohort of patients with acromegaly. Furthermore, the study aimed to confirm the long-term efficacy and safety of Lan-Autogel.
Methods
Patients
All patients were required to be at least 18 years old and have active acromegaly. Patients who had never received a somatostatin analogue (‘Naive’) or a dopamine agonist, or had stopped taking this medication more than 3 months before (‘Not Treated within 3 months’), were eligible if their mean serum GH level was >5 ng/ml at screening. Patients who were receiving a somatostatin analogue or a dopamine agonist immediately prior to the study (‘Previously Treated’) underwent treatment washout until their mean GH was >3 ng/ml, and had increased by at least 100% from screening values. The duration of washout depended on the dosing interval of the previous drug: GH and insulin-like growth factor-1 (IGF-1) levels were assessed after two dosing intervals had elapsed since the last injection. If patients still did not meet the inclusion criteria, a second assessment was allowed 14–42 days later. Patients were excluded if they had received radiotherapy for acromegaly within 3 years or pituitary surgery within 3 months prior to screening, or anticipated requiring such treatment during the study period. The use of Lan-Autogel or a GH receptor antagonist at any time before the study, were also exclusion criteria, as were pregnancy, breastfeeding, or hepatic or renal impairment. Somatostatin analogues (other than Lan-Autogel), dopamine agonists or cyclosporine (due to the risk of reduced absorption when co-administered with Lan-Autogel) were not permitted during the study. The study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki and with the laws and regulations of the countries in which the research was conducted, whichever afforded the greater protection to the individual. The investigators adhered to the provisions set out in Good Clinical Practice Guidelines.
Study design
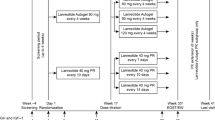
Lan-Autogel was administered every 28 days by deep subcutaneous injection for 13 injections (Fig. 1). The study was conducted in four phases: the first was a washout phase for previously treated patients, followed by a double-blind phase, in which patients were randomized to one injection of Lan-Autogel 60, 90 or 120 mg or placebo. In the following single-blind phase, patients received three further injections at the same dose, and the placebo group was re-allocated to Lan-Autogel 60, 90 or 120 mg. In the final, open-label phase, doses could be titrated according to biochemical response, as assessed 28 days after the fifth and ninth injections (Fig. 1). At each titration step, the dose could increase or decrease by only one dose level (30 mg) and, once increased, the dose could not subsequently be reduced.
Study design showing the dose-titration schema. Dose-titration was based on serum levels of growth hormone (GH; ng/ml) and whether insulin-like growth factor-1 (IGF-1) levels were in the age-adjusted normalized range (N); A = GH > 2.5 or GH ≤ 2.5 + IGF-1 > N; B = GH ≤ 2.5 + IGF-1 ≤ N; C = 1 < GH ≤ 2.5 + IGF-1 ≤ N; D = GH ≤ 1 + IGF-1 ≤ N; E = GH > 1 or GH ≤ 1 + IGF-1 > N. *Only if 60 mg originally
Efficacy and safety assessments
Mean GH levels were determined from a series of seven serum samples obtained at 30-min intervals at screening and before each injection at weeks 4, 13, 14, 15, 16, 32 and 52, or at the time of early withdrawal. A single serum sample was obtained for the assessment of IGF-1 levels at the same visits. Both GH and IGF-1 concentrations were measured using Nichols Advantage Chemiluminescence assays (Nichols Institute Diagnostics, CA, USA) at a single center. The limits of detection and quantitation of the GH assay were 0.01 and 0.15 ng/ml, respectively, intra-assay coefficient of variation was 2.6–7.9%, and inter-assay coefficient of variation was 4.7–6.6%. For the IGF-1 assay, the limits of detection and quantitation were 0.8 and 2 nmol/l, respectively, the intra-assay variability was 4.9–7.9% and the inter-assay variability was 5.0–8.7%. Serum samples from weeks 0, 4, 16, 36 and 52 were assessed for non-specific binding (NSB) to lanreotide, and those which showed >30% NSB were assessed for the presence of antibodies against lanreotide. This was achieved by comparing the displacement obtained with lanreotide, octreotide and somatostatins 14 and 28. Acromegaly symptoms were assessed by the investigator during screening and at weeks 0, 4, 16, 32 and 52; standard biochemistry and haematology assessments, physical examinations and electrocardiograms (ECGs) were made at weeks 0, 4 16 and 52. Echocardiography and gall-bladder ultrasound were performed at weeks 0, 16 and 52. A retrospective centralized analysis of ECG and echocardiography data was conducted and the data reviewed by a cardiologist. Adverse events were recorded at each study visit.
Statistical analysis
The primary efficacy endpoint was the proportion of patients with a >50% reduction in mean GH levels from baseline to week 4. Each Lan-Autogel dose-group was compared with placebo, as was the pooled Lan-Autogel group (Lan-Autogel All). An exploratory analysis of the primary efficacy endpoint was performed, stratified by geographic region (USA and Europe plus Hong Kong) using the Cochran-Mantel-Haenszel test (for the treatment effect) and the Breslow-Day test (for the region by treatment interaction). Secondary efficacy endpoints included the effect of repeated Lan-Autogel injections on GH and IGF-1 levels at specific time points: the proportions of patients with >50% reduction in GH from baseline, GH ≤ 2.5 ng/ml, or normalized IGF-1 or both normalized IGF-1 and GH ≤ 2.5 ng/ml. The assessment of acromegaly symptoms between baseline and weeks 4, 16, 32 and 52, or the end of the study, was also an endpoint.
The analysis of categorical efficacy data was performed using the Cochran-Mantel-Haenszel test, with the use of the Mantel-Haenszel or the logit estimation of the odds ratios, using region as a stratification variable. Changes in continuous efficacy variables from baseline were analyzed by ANOVA, using the baseline value as a covariate. The primary efficacy endpoint was adjusted for multiple comparisons using Fisher’s exact test with permutation re-sampling. The intention-to-treat population was the primary population for all efficacy analyses. Post hoc analyses were performed to calculate treatment response (percentage of patients with GH ≤ 2.5 ng/ml; percentage of patients with normalized IGF-1 and percentage of patients meeting both of these criteria) by the following baseline characteristics: sex, age, race, severity of acromegaly, previous drug treatment, history of radiotherapy and surgery. These were calculated as mean percentage of patients and 95% confidence intervals (CI).
Results
Patients
Patients were recruited at 30 centers in eight countries across the USA, Europe and Hong Kong (Appendix). A total of 220 patients were screened and 111 randomized (Fig. 2). Study drug was received by 108 patients in the double-blind phase; 50% were classified as Naive (n = 15) or Not Treated within 3 months (n = 39), and 50% as Previously Treated (n = 54). Of these, 107 patients continued to the single-blind phase (one patient withdrew because of an adverse event ongoing from screening); 105 patients completed the double-blind phase and entered the open-label dose-titration phase (two withdrawals were caused by adverse events not related to treatment). The study was completed by 99 patients (four patients withdrew during the open-label phase because of a lack of efficacy and two because of adverse events [growth of a pre-existing meningioma in one patient and albuminuria, diabetic nephropathy and peripheral oedema in the other]). At the end of the single-blind phase, the final dose of Lan-Autogel was 60 mg for 34 patients, 90 mg for 36 patients and 120 mg for 37 patients; the number of patients on each dose at the end of the open-label phase was 21, 15 and 69, respectively.
Baseline characteristics and pre-treatment status were similar among dose groups (Table 1), except that proportionally more Naive and Not Treated within 3 months patients were randomized to the 60 mg group than the other dosing groups.
First injection
Four weeks after the first injection, more patients showed a >50% reduction in GH level with Lan-Autogel than with placebo (63, 52, 44, 90 and 0% of patients for Lan-Autogel All, 60, 90, 120 mg and placebo, respectively). All differences between the lanreotide groups and placebo were statistically significant (P < 0.001). No patients had GH levels ≤ 2.5 ng/ml at baseline (before the start of the double-blind period). Four weeks after the first injection, however, 34% (28/83) of patients in the Lan-Autogel All group had this level of GH control, compared with none (0/25) in the placebo group (P < 0.001). Ten percent (8/83) of the patients receiving Lan-Autogel had normal IGF-1 levels at baseline, and the proportion increased to 25% (21/83) after one injection; 8% (2/25) of the placebo group had normal IGF-1 levels at baseline, which decreased to 4% (1/25) 4 weeks after the first injection (P < 0.05 vs. Lan-Autogel All group). The combined criterion of GH levels ≤ 2.5 ng/ml and normalized IGF-1 levels was met by 16% (13/83) of patients in the Lan-Autogel All group and 0% in the placebo group, 4 weeks after the first injection. Overall, mean (SD) GH concentrations declined from 20.3 (30.0) to 8.1 (13.5) ng/ml at week 4 and mean (SD) IGF-1 concentrations from 744 (236) to 532 (262) ng/ml in the Lan-Autogel All group. These reductions were statistically significant compared with the placebo group (mean GH increased from 18.2 to 22.5 ng/ml, and mean IGF-1 increased from 702 to 733 ng/ml; P < 0.001 vs. Lan-Autogel groups). Analysis by geographical region showed a similar response to treatment for patients from the USA and those from Europe/Hong Kong.
Fixed-dose phase
After a further three injections of Lan-Autogel in the fixed-dose phase, control of GH and IGF-1 levels continued to improve. At week 16, 72% of patients had a >50% reduction in GH levels from baseline, 49% had GH levels ≤ 2.5 ng/ml and 54% had normalized IGF-1 levels in the Lan-Autogel All group (Fig. 3a). An indication of dose responsiveness was apparent for >50% reduction in GH levels: a slightly higher proportion showed a response in the 120 mg Lan-Autogel group compared with the 90 and 60 mg groups but the difference was not statistically significant (P = 0.056 for 120 vs. 90 mg and P = 0.116 for 120 vs. 60 mg). Overall, mean GH levels decreased by 62.3% (SD 36.6%; median reduction was 75.5%) from baseline to week 16 with Lan-Autogel treatment (P < 0.001), and the highest dose produced significantly greater reductions than the lowest dose (mean [sd] for 60 mg 53.0% [42.2%] vs. 120 mg 72.1% [33.0%]; P = 0.022).
At week 16, mean IGF-1 levels decreased from baseline. Similarly for IGF-1, there was an indication of dose responsiveness with a significant difference between doses for the 60 mg (mean [SD] reduction of 32% [38%]) vs. the 120 mg group (54% [19%]; P = 0.002). There was a similar response to treatment after 16 weeks across the different geographical regions.
Dose-titration phase
By week 52, 82% of all patients (Lan-Autogel All) had a mean decrease in serum GH levels of >50% from baseline and 54% had mean GH levels ≤ 2.5 ng/ml; 59% of patients had normalized IGF-1 levels, and 43% had both GH levels ≤ 2.5 ng/ml and normalized IGF-1 levels (Fig. 3b). GH and IGF-1 levels were significantly reduced from baseline at week 52, with mean (SD) reductions of 67.1% (32.0%; P < 0.001) and 48.9% (28.6%), respectively (P < 0.001; Fig. 4). According to the dosing scheme, patients who were less responsive were given higher doses; mean GH and IGF-1 levels were higher and the proportion of patients with GH ≤ 2.5 ng/ml and normalized IGF-1 was lower in the 120 mg group than the 60 mg group. In patients not requiring dose escalation to 120 mg, 85% (29 of 34 patients) achieved biochemical control (normalized IGF-1 levels and GH < 2.5 ng/ml).
Subset analyses
At week 16 (the end of the fixed-dose phases), the proportions of patients who were Naive or Not Treated within 3 months and Previously Treated in each dose group were reasonably balanced, despite not having been stratified for prior treatment. By week 52, most of the patients who were Naive or Not Treated within 3 months (82%) were receiving the 120 mg dose, whereas 49% of Previously Treated patients were receiving this dose. Most baseline variables did not appear to affect response to treatment (Table 2). However patients with less severe acromegaly at baseline (GH < 10 ng/ml) showed a better treatment response (mean GH ≤ 2.5 ng/ml) than those with more severe disease (Table 2) and patients with prior pituitary surgery also appeared to respond better.
Symptoms
Compared with baseline, acromegaly symptoms improved or were stable in most patients. Between baseline and week 16, perspiration was improved or stable in 94% of patients, oedema of extremities in 93%, joint pain in 92%, fatigue in 92%, headache in 94%, impotence in 94% and oligomenorrhoea in 91%. By week 52, perspiration was improved or stable in 93% of patients, oedema of extremities in 94%, joint pain in 87%, fatigue in 90%, headache in 88%, impotence in 89% and oligomenorrhoea in 100%.
Safety
Across all three study phases, 98 of the 107 patients (92%) who received Lan-Autogel, experienced at least one adverse event. The most common adverse events were gastrointestinal in nature and their incidence increased with dose (Table 3). The majority of adverse events were rated as mild to moderate; 30% (32/107) of patients experienced severe adverse events. Severe events were reported in 15% (7/46) patients during treatment with Lan-Autogel 60 mg, 17% (11/66) patients during treatment with Lan-Autogel 90 mg and 23% (17/74) patients with Lan-Autogel 120 mg (note: any of the 32 patients experiencing a severe adverse event could be counted more than once in these calculations if they experienced a severe adverse event on more than one dose of Lan-Autogel). The most commonly reported adverse events rated as severe were: abdominal pain (7 patients, 7%), diarrhoea (6 patients, 6%), and hypertension aggravated (3 patients, 3%). Other severe adverse events were reported in no more than two patients. Serious adverse events occurred in 17% (18/107) of patients receiving Lan-Autogel, and in one patient (4%) receiving placebo. Only one serious event, pancreatitis associated with gall bladder lithiasis migration, was considered related to study treatment. No deaths occurred during the study. One patient withdrew because of an adverse event ongoing from screening, and three patients receiving 90 mg and one receiving 120 mg of Lan-Autogel withdrew because of adverse events (fracture, thyroid carcinoma, diabetic nephropathy, growth of pre-existing meningioma), but none was assessed as drug-related.
No clinically meaningful changes in haematology, chemistry or vital signs were noted following single or repeated injections of Lan-Autogel. There were no clinically important changes in blood glucose levels and HbA1c in any treatment group at weeks 4, 16 and 52. Gallstones and/or sludge were already present in 30% (30/100) of patients at baseline (19/100 with gallstones and 14/100 with sludge; three patients had both). In total, 22 patients (26%) whose baseline ultrasound was free of abnormalities had newly identified gallstones or sludge by the end of the study.
For most patients (72% [73/101]), ECG assessments were normal and remained normal during the study; 22 patients had abnormal ECGs both at baseline and at the end of the study and five patients shifted from normal to abnormal. Across all patients, heart rate decreased by a mean (SD) of 8.4 (14.8) bpm and mean ECG intervals changed accordingly. Although all changes from baseline were statistically significant, they were within clinically accepted normal limits. Echocardiography measurements showed that no major modifications occurred during the study, other than a decrease in left ventricular mass, which paralleled the change in body size. Concordant with this, mean (SD) left ventricular end-diastolic and -systolic volumes decreased by 9.9 (20.3) and 5.4 (16.1) ml, respectively. Valvular regurgitations were typical of patients with acromegaly and did not show any clinically meaningful changes over time.
The presence of putative antibodies to Lan-Autogel was observed in one patient in two samples (at baseline and after the first injection). However, no assessment of putative antibodies could be found in later samples; this patient had normalized IGF-1 levels by week 16 and had no allergic-type reaction. The only drug-related adverse event reported for this patient was diarrhoea at week 15.
Discussion
This 52-week, four-phase study in a large international cohort of unselected patients with acromegaly has shown a sustained control of GH and IGF-1 levels with 28-day Lan-Autogel treatment. The design of the trial allowed a double-blind comparison with placebo for a single, randomized dose of Lan-Autogel 60, 90 or 120 mg. These doses were continued in the fixed-dose phase for a further four injections (with placebo-patients re-allocated) allowing investigation of dose-responsiveness. Doses were tailored according to biochemical response in the open-label titration phase, which mimicked actual clinical practice, with the exception that once doses were increased, they could not then be reduced.
The primary efficacy outcome, a >50% reduction in mean GH levels from baseline, was selected to show clearly the different responses to Lan-Autogel and placebo, 4 weeks after a single injection. This reduction in GH levels was achieved in 63% of patients receiving Lan-Autogel but not in any patients receiving placebo. As steady-state levels of lanreotide were achieved after 3–4 injections [20], the proportion of patients with mean GH ≤ 2.5 ng/ml increased, reaching 49% of all patients at week 16 and 54% at week 52. IGF-1 control also improved: 59% of patients had age-adjusted normalized levels by week 52 and 43% achieved control of both the GH and IGF-1 parameters by week 52.
It is notable that patients who had previously received a somatostatin analogue, or a dopaminergic agonist, had a significantly enhanced response rate than patients who were Naive or Not Treated within 3 months of the study. At the end of the study, 69% of Previously Treated patients had GH levels ≤ 2.5 ng/ml. The corresponding proportion was 38.5% for patients Not Treated within previous 3 months and 33% for truly drug-naive patients. A similar effect was seen for normalized IGF-1 levels, the corresponding values were 71, 49 and 40% at week 52, respectively. This observation is readily explained if we consider that patients who gained benefit from treatment with somatostatin analogues were more likely to have continued such therapy up to study entry, whereas those who had previously not experienced a treatment response were more likely to have discontinued therapy before the study. The population of drug-naive patients would not be biased in this way towards any treatment response.
Less severe acromegaly at baseline was easier to treat than more severe disease, which was reflected in greater treatment responses at endpoint. Not all studies of somatostatin analogues are in agreement with this observation, for example, work by Cozzi et al. with octreotide shows no relationship between response and baseline levels of GH or IGF-1 [22, 23]. One limitation of this study was the absence of adenoma size measurements that could have provided information on tumor shrinkage.
In addition, prior pituitary surgery tended to predispose patients to a better treatment response to Lan-Autogel, a finding that has also been reported for the lanreotide microparticle formulation [24]. There were too few patients having previously undergone radiotherapy to confidently assess its effect on response to lanreotide.
Natural variations in responsiveness to somatostatin analogues among the population as a whole is well established, and is thought to arise from differing expressions of somatostatin receptor subtypes, notably receptors 2 and 5 [25, 26].
The efficacy of Lan-Autogel demonstrated in the total population of the present study is comparable with results previously published for octreotide [27]. Indeed, the efficacy outcomes of the present study are almost identical to those from the study of Cozzi et al. [22]. Twelve months’ treatment with octreotide long-acting repeatable (LAR), achieved GH levels < 2.5 ng/ml in 54% of patients and normalized IGF-1 in 61%. This similarity in efficacy between the two somatostatin analogues has been demonstrated in many comparative studies [28–31]. A review conducted in 2003 concluded that in unselected patients octreotide LAR was more effective than slow release lanreotide for reducing GH and IGF-1 levels [32], but a recent review of the literature reporting the efficacy of lanreotide and octreotide LAR demonstrated that Lan-Autogel and octreotide LAR were equivalent in both biological and symptom control in patients with acromegaly [17]. The recently updated guidelines on acromegaly management also state that octreotide LAR and Lan-Autogel have equivalent efficacy [33].
At the end of the fixed-dose phase, an indication of dose-responsiveness was apparent. The decline in mean GH and IGF-1 levels was greater, and the proportions of patients achieving control tended to be higher, amongst the group randomized to 120 mg than the lower dose groups. This dose-responsiveness was reversed during the dose-titration phase, as patients who responded less well to treatment underwent dose titration to higher doses of Lan-Autogel.
Treatment with Lan-Autogel was generally well tolerated and the incidence of adverse events was low. This is reflected by the large proportion of patients who remained in the study: 99 of 111 patients randomized to treatment completed the study and there were no withdrawals as a result of treatment-related adverse events. The most common adverse events were, as expected, gastrointestinal in nature. As is seen with all somatostatin-analogue therapies, there was an increase in the incidence of gall-bladder lithiasis and sludge over the course of the study. However, cardiac findings were unremarkable and all changes would have been expected in this patient population. Administration of Lan-Autogel by deep subcutaneous injection was shown to have negligible immunogenic impact, with putative antibodies against lanreotide detected in only one patient.
In summary, Lan-Autogel was effective in controlling GH and IGF-1 hypersecretion in patients with acromegaly and showed a rapid onset of action. It was well tolerated and the safety profile was as expected for a somatostatin analogue. The results support the concept that the deep subcutaneous route is both safe and effective compared with deep intramuscular injections, and the 28-day dosing regimen met with high patient compliance. These results further support the use of Lan-Autogel for the long-term treatment of acromegaly.
References
Melmed S (2006) Medical progress: acromegaly. N Engl J Med 355:2558–2573
Alexander L, Appleton D, Hall R, Ross WM, Wilkinson R (1980) Epidemiology of acromegaly in the Newcastle region. Clin Endocrinol (Oxf) 12:71–79
Bates AS, Van’t Hoff W, Jones JM, Clayton RN (1993) An audit of outcome of treatment in acromegaly. Q J Med 86:293–299
Holdaway IM, Rajasoorya C (1999) Epidemiology of acromegaly. Pituitary 2:29–41
Rajasoorya C, Holdaway IM, Wrightson P, Scott DJ, Ibbertson HK (1994) Determinants of clinical outcome and survival in acromegaly. Clin Endocrinol (Oxf) 41:95–102
Maiza JC, Vezzosi D, Matta M, Donadille F, Loubes-Lacroix F, Cournot M, Bennet A, Caron P (2007) Long-term (up to 18 years) effects on GH/IGF-1 hypersecretion and tumour size of primary somatostatin analogue (SSTa) therapy in patients with GH-secreting pituitary adenoma responsive to SSTa. Clin Endocrinol (Oxf) 67:282–289
Melmed S, Casanueva FF, Cavagnini F et al (2002) Guidelines for acromegaly management. J Clin Endocrinol Metab 87:4054–4058
Baldelli R, Colao A, Razzore P et al (2000) Two-year follow-up of acromegalic patients treated with slow release lanreotide (30 mg). J Clin Endocrinol Metab 85:4099–4103
Caron P, Morange-Ramos I, Cogne M, Jaquet P (1997) Three year follow-up of acromegalic patients treated with intramuscular slow-release lanreotide. J Clin Endocrinol Metab 82:18–22
Heron I, Thomas F, Dero M, Gancel A, Ruiz JM, Schatz B, Kuhn JM (1993) Pharmacokinetics and efficacy of a long-acting formulation of the new somatostatin analog BIM 23014 in patients with acromegaly. J Clin Endocrinol Metab 76:721–727
Johnson MR, Chowdrey HS, Thomas F, Grint C, Lightman SL (1994) Pharmacokinetics and efficacy of the long-acting somatostatin analogue somatuline in acromegaly. Eur J Endocrinol 130:229–234
Caron P, Beckers A, Cullen DR, Goth MI, Gutt B, Laurberg P, Pico AM, Valimaki M, Zgliczynski W (2002) Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegaly. J Clin Endocrinol Metab 87:99–104
Caron P, Bex M, Cullen DR, Feldt-Rasmussen U, Pico Alfonso AM, Pynka S, Racz K, Schopohl J, Tabarin A, Valimaki MJ (2004) One-year follow-up of patients with acromegaly treated with fixed or titrated doses of lanreotide Autogel. Clin Endocrinol (Oxf) 60:734–740
Caron P, Cogne M, Raingeard I, Bex-Bachellerie V, Kuhn JM (2006) Effectiveness and tolerability of 3-year lanreotide Autogel treatment in patients with acromegaly. Clin Endocrinol (Oxf) 64:209–214
Chanson P, Borson-Chazot F, Kuhn JM, Blumberg J, Maisonobe P, Delemer B (2008) Control of IGF-I levels with titrated dosing of lanreotide Autogel over 48 weeks in patients with acromegaly. Clin Endocrinol (Oxf) 69:299–305
Croxtall JD, Scott LJ (2008) Lanreotide autogel(r): a review of its use in the management of acromegaly. Drugs 68:711–723
Murray RD, Melmed S (2008) A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab 93:2957–2968
Lucas T, Astorga R (2006) Efficacy of lanreotide Autogel administered every 4–8 weeks in patients with acromegaly previously responsive to lanreotide microparticles, 30 mg: a phase III trial. J Clin Endocrinol Metab 65:320–326
Antonijoan RM, Barbanoj MJ, Cordero JA, Peraire C, Obach R, Valles J, Cherif-Cheikh R, Torres ML, Bismuth F, Montes M (2004) Pharmacokinetics of a new Autogel formulation of the somatostatin analogue lanreotide after a single subcutaneous dose in healthy volunteers. J Pharm Pharmacol 56:471–476
Bronstein M, Musolino N, Jallad R, Cendros JM, Ramis J, Obach R, Leselbaum A, Catus F (2005) Pharmacokinetic profile of lanreotide Autogel in patients with acromegaly after four deep subcutaneous injections of 60, 90 or 120 mg every 28 days. Clin Endocrinol (Oxf) 63:514–519
Cendros JM, Peraire C, Troconiz IF, Obach R (2005) Pharmacokinetics and population pharmacodynamic analysis of lanreotide Autogel. Metabolism 54:1276–1281
Cozzi R, Attanasio R, Montini M, Pagani G, Lasio G, Lodrini S (2003) Four-year treatment with octreotide long-acting repeatable in 110 acromegalic patients: predictive value of short-term results. J Clin Endocrinol Metab 88:3090–3098
Cozzi R, Montini M, Attanasio R, Albizzi M, Lasio G, Lodrini S, Doneda P, Cortesi L, Pagani G (2006) Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkage. J Clin Endocrinol Metab 91:1397–1403
Karavitaki N, Turner HE, Adams CB, Cudlip S, Byrne JV, Fazal-Sanderson V, Rowlers S, Trainer PJ, Wass JA (2008) Surgical debulking of pituitary macroadenomas causing acromegaly improves control by lanreotide. Clin Endocrinol (Oxf) 68:970–975
Culler MD, Taylor JE, Moreau JP (2002) Somatostatin receptor subtypes: targeting functional and therapeutic specificity. Ann Endocrinol (Paris) 63:2S5–2S12
Jaquet P, Saveanu A, Gunz G et al (2000) Human somatostatin receptor subtypes in acromegaly: distinct patterns of messenger ribonucleic acid expression and hormone suppression identify different tumoral phenotypes. J Clin Endocrinol Metab 85:781–792
Mercado M, Borges F, Bouterfa H et al (2007) A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR® (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol 66:859–868
Alexopoulou O, Adams P, Verhelst J, Poppe K, Velkeniers B, Abs R (2004) Efficacy and tolerability of lanreotide Autogel therapy in acromegalic patients previously treated with octreotide LAR. Eur J Endocrinol 151:317–324
Amato G, Mazziotti G, Rotondi M et al (2002) Long-term effects of lanreotide SR and octreotide LAR on tumour shrinkage and GH hypersecretion in patients with previously untreated acromegaly. Clin Endocrinol (Oxf) 56:65–71
Ronchi CL, Boschetti M, Degli Uberti EC et al (2007) Efficacy of a slow-release formulation of lanreotide (Autogel) 120 mg) in patients with acromegaly previously treated with octreotide long acting release (LAR): an open, multicentre longitudinal study. Clin Endocrinol (Oxf) 67:512–519
van Thiel SW, Romijn JA, Biermasz NR, Ballieux BE, Frolich M, Smit JW, Corssmit EP, Roelfsema F, Pereira AM (2004) Octreotide long-acting repeatable and lanreotide Autogel are equally effective in controlling growth hormone secretion in acromegalic patients. Eur J Endocrinol 150:489–495
Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D (2005) Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab 90:4465–4473
Melmed S, Colao A, Barkan A et al (2009) Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 94:1509–1517
Acknowledgments
The authors take full responsibility for the content of the paper, and thank Caudex Medical and ESP Bioscience (supported by Ipsen) for their assistance in preparation of the manuscript and its revision addressing the authors’ comments. M. Goth is supported by an OTKA Grant (no. 68660).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The Global Study Group for Lanreotide Autogel® in Acromegaly comprises the following investigators, who conducted this study: S. Melmed (USA), A. Barkan (USA), D. Cook (USA), D. Kleinberg (USA), L. Katznelson (USA), M. Molitch (USA), P. Snyder (USA), P. Kim (USA), E. Ennis (USA), M. Sharma (USA), G. Weryha (France), D. Dewailly (France), P. Jaquet (France), A. Warnet (France), X. Bertagna (France), H. Gerl (Germany), J. Schopohl (Germany), K. Mann (Germany), P. Stewart (UK), P. Bouloux (UK), R. Salvatori (USA), J. Marek (Czech Republic), J. Cap (Czech Republic), J. Mertl (Czech Republic), W. de Herder (The Netherlands), A. Arias (The Netherlands), K. Racz (Hungary), M. Goth (Hungary), K. Lam (Hong Kong), P. Trainer (UK).
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Melmed, S., Cook, D., Schopohl, J. et al. Rapid and sustained reduction of serum growth hormone and insulin-like growth factor-1 in patients with acromegaly receiving lanreotide Autogel® therapy: a randomized, placebo-controlled, multicenter study with a 52 week open extension. Pituitary 13, 18–28 (2010). https://doi.org/10.1007/s11102-009-0191-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-009-0191-1