Abstract
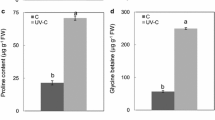
The effects of enhanced ultraviolet-B (UV-B, 0.4 W m−2) irradiance and nickel (Ni, 0.01, 0.10 and 1.00 mM; Ni0.01, Ni0.10, Ni1.00, respectively) treatment, singly and in combination, on growth, photosynthetic electron transport activity, the contents of reactive oxygen species (ROS), antioxidants, lipid peroxidation, and membrane leakage in soybean seedlings were evaluated. Ni0.10 and Ni1.00 and UV-B declined the growth and chlorophyll content, which were further reduced following combined exposure. Contrary to this, Ni0.01 stimulated growth, however, the effect together with UV-B was inhibitory. Carotenoids showed varied response to both the stresses. Simultaneous exposure of UV-B and Ni as well as UV-B alone reduced the activities of photosystems 1 and 2 (PS1 and PS2) and whole chain activity significantly, while Ni individually, besides strongly inhibiting PS2 and whole chain activity, stimulated the PS1 activity. Both the stresses, alone and together, enhanced the contents of superoxide radical (O ⋅−2 ), hydrogen peroxide (H2O2), malondialdehyde (MDA), electrolyte leakage, and proline content, while ascorbate content declined over control. Individual treatments increased the activities of catalase (CAT), peroxidase, and superoxide dismutase (SOD), but Ni1.00 declined SOD activity significantly. Combined exposure exhibited similar response, however, CAT activity declined even more than in control. Compared to individual effects of UV-B and Ni, the simultaneous exposure resulted in strong inhibition of photosynthetic electron transport and excessive accumulation of ROS, thereby causing severe damage to soybean seedlings.
Similar content being viewed by others
Abbreviations
- ASC:
-
ascorbate
- CAT:
-
catalase
- Chl:
-
chlorophyll
- DCMU:
-
3-(3,4 dichlorophenyl)-1,1-dimethyl urea
- DCPIP:
-
2,6-dichlorophenol indophenol
- MDA:
-
malondialdehyde
- MV:
-
methyl viologen
- NBT:
-
p-nitroblue tetrazolium chloride
- p-BQ:
-
p-benzoquinone
- PPFD:
-
photon flux density
- POD:
-
peroxidase
- PS:
-
photosystem
- SOD:
-
superoxide dismutase
- UV-B:
-
ultraviolet-B radiation (280–320 nm).
References
Almog, O., Lotan, O., Shoham, G., Nechushtai, R.: The composition and organization of photosystem I. — J. basic clin. Physiol. Pharmacol. 2: 123–140, 1991.
Arnon, D.I.: Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. — Plant Physiol. 24: 1–15, 1949.
Arora, A., Sairam, R.K., Srivastava, G.C.: Oxidative stress and antioxidant system in plants. — Curr. Sci. 82: 1227–1238, 2002.
Bates, L.S., Waldren, R.P., Teare, I.D.: Rapid determination of free proline for water stress studies. — Plant Soil 39: 205–207, 1973.
Brown, P.H., Welch, R.M., Cary, E.E.: Nickel: A micronutrient essential for higher plants. — Plant Physiol. 85: 801–803, 1987.
Caldwell, M.M., Bjorn, L.O., Bornman, J.F., Flint, S.D., Kulandaivelu, G., Teramura, A.H., Tevini, M.: Effects of increased solar ultraviolet radiation on terrestrial ecosystems. — J. Photochem. Photobiol. 46: 40–52, 1998.
Carlson, R.W., Bazzaz, F.A., Rolf, G.L.: The effect of heavy metals on plants II. Net photosynthesis and transpiration of whole corn and sunflower plants treated with Pb, Cd, Ni, and Ti. — Environ. Res. 10: 113–120, 1975.
Dai, Q., Yan, B., Huang, S., Liu, X., Peng, S., Miranda, M.L.L., Chavez, A.Q., Vergara, B.S., Olszyk, D.M.: Response of oxidative stress defense systems in rice (Oryza sativa) leaves with supplemental UV-B radiation. — Physiol. Plant. 101: 301–308, 1997.
Devine, M., Duke, S.O., Fedtke, C.: Physiology of Herbicide Action. — PTR Prentice-Hall, Englewood Cliffs 1993.
Dube, S.L., Bornman, J.F.: Response of spruce seedlings to simultaneous exposure to ultraviolet-B radiation and cadmium. — Plant Physiol. Biochem. 30: 761–767, 1992.
Egashira, T., Takahama, U., Nakamura, K.: A reduced activity of catalase as a basis for light dependent methionine sensitivity of a Chlamydomonas reinhardtii mutant. — Plant Cell Physiol. 30: 1171–1175, 1989.
Elstner, E.F., Heupel, A.: Inhibition of nitrite formation from hydroxylammonium chloride: A simple assay for superoxide dismutase. — Anal. Biochem. 70: 616–620, 1976.
Gabrielli, R., Mattioni, C., Vergnano, O.: Accumulation mechanism and heavy metal tolerance of nickel hyperaccumulator. — J. Plant Nutr. 14: 1067–1080, 1991.
Gahagen, H.E., Holm, R.E., Abeles, F.B.: Effect of ethylene on peroxidase activity. — Physiol. Plant. 21: 1270, 1968.
Giannopolitis, C.N., Ries, S.K.: Superoxide dismutases. I. Occurrence in higher plants. — Plant Physiol. 59: 309–314, 1977.
Gong, M., Li, Y.J., Chen, S.Z.: Abscisic acid induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. — J. Plant Physiol. 153: 488–496, 1998.
Goodwin, T.W.: Carotenoids. — In: Paech, K., Tracey, M.W. (ed.): Handbook of Plant Analysis. Pp. 272–311. Springer-Verlag, Berlin 1954.
Gopal, R., Mishra, K.B., Zeeshan, M., Prasad, S.M., Joshi, M.M.: Laser induced chlorophyll fluorescence spectra of mung plants growing under nickel stress. — Curr. Sci. 83: 880–884, 2002.
Heath, R.L., Packer, L.: Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. — Arch. Biochem. Biophys. 125: 189–198, 1968.
Hideg, E., Vass, I.: UV-B induced free radical production in plant leaves and isolated thylakoid membranes. — Plant Sci. 115: 251–260, 1996.
Jansen, M.A.K., Gaba, V., Greenberg, B.M.: Higher plants and UV-B radiation: balancing, damage, repair and acclimation. — Trends Plant Sci. 3: 131–135, 1998.
Jordan, B.R., Chow, W.S., Strid, A., Anderson, J.M.: Reduction in cab and psbA RNA transcripts in response to supplemental ultraviolet-B radiation. — FEBS Lett. 284: 5–8, 1991.
Kondo, N., Kawashima, M.: Enhancement of the tolerance to oxidative stress in cucumber (Cucumis sativus L.) seedlings by UV-B irradiation: Possible involvement of phenolic compounds and antioxidative enzymes. — J. Plant Res. 113: 311–317, 2000.
Krupa, Z., Skorzynska, E., Maksymiec, W., Baszynski, T.: Effect of cadmium treatment on the photosynthetic apparatus and its photochemical activities in greening radish seedlings. — Photosynthetica 21: 156–164, 1987.
Kulandaivelu, G., Maragatham, S., Nedunchezhian, N.: On the possible control of ultraviolet-B induced response in growth and photosynthetic activities in higher plants. — Physiol. Plant. 76: 398–404, 1989.
Kupper, H., Kupper, F., Spiller, M.: Environmental relevance of heavy metal substituted chlorophylls using the example of water plants. — J. exp. Bot. 47: 259–266, 1996.
Lingakumar, K., Kulandaivelu, G.: Changes induced by ultraviolet-B radiation in vegetative growth, foliar characteristics and photosynthetic activities in Vigna unguiculata. — Aust. J. Plant Physiol. 20: 299–308, 1993.
Madronich, S., Mckenzie, L.O., Bjorn, L.O., Caldwell, M.M.: Changes in biologically active ultraviolet radiation reaching the Earth’s surface. — J Photochem. Photobiol. B 46: 5–1, 1998.
Mahalingam, R., Fedoroff, N.: Stress response, cell death and signaling: The many faces of reactive oxygen species. — Physiol. Plant. 119: 56–68, 2003.
Matysik, J., Alia, Bhalu, B., Mohanty, P.: Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. — Curr. Sci. 82: 525–532, 2002.
Mohanty, N., Vass, I., Demeter, S.: Impairment of photosystem 2 activity at the level of secondary quinone electron acceptor in chloroplasts treated with cobalt, nickel and zinc ions. — Physiol. Plant. 76: 386–390, 1989.
Oser, B.L.: Hawks Physiological Chemistry. — Pp. 702–705. McGraw Hill, New York 1979.
Ouzounidou, G.: Cu-ions mediated changes in growth, chlorophyll and other ion contents in a Cu-tolerant Koeleria splendens. — Biol. Plant. 37: 71–78, 1995.
Pandolfini, T., Gabbrielli, R., Comparini, C.: Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. — Plant Cell Environ. 15: 719–725, 1992.
Pardha Saradhi, P., Alia, Arora, S., Prasad, K.V.S.K.: Proline accumulates in plants exposed to UV-radiation and protects them against UV-induced peroxidation. — Biochem. biophys. Res. Commun. 209: 1–5, 1995.
Porra, R.J.: The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. — Photosynth. Res. 73: 149–156, 2002.
Rai, L.C., Tyagi, B., Mallick, N., Rai, P.K.: Interactive effects of UV-B and copper on photosynthetic activity of the cyanobacterium Anabaena doliolum. — Environ. exp. Bot. 35: 177–185, 1995.
Rai, L.C., Tyagi, B., Rai, P.K., Mallick, N.: Interactive effects of UV-B and heavy metals (Cu and Pb) on nitrogen and phosphorous metabolism of a N2-fixing cyanobacterium Anabaena doliolum. — Environ. exp. Bot. 39: 221–231, 1998.
Rao, M.V., Paliyath, G., Ormrod, D.P.: Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. — Plant Physiol. 110: 125–136, 1996.
Renger, G., Volker, M., Eckert, H.J., Fromme, R., Hohm-Veit, S., Graber, P.: On the mechanism of photosystem II deterioration by UV-B irradiation. — Photochem. Photobiol. 49: 97–105, 1989.
Sagisaka, S.: The occurrence of peroxide in a perennial plant Populas gelrica. — Plant Physiol. 57: 308–309, 1976.
Srivastava, A., Tel, O.E.: Antioxidative enzymatic response of Lemna to environmental pollutants. — J. environ. Sci. Health A 27: 261–272, 1992.
Strid, A., Chow, W.S., Anderson, J.M.: Effects of supplementary ultraviolet-B radiation on photosynthesis in Pisum sativum. — Biochim. biophys. Acta 1020: 260–268, 1990.
Strid, A., Porra, R.J.: Alterations in pigment content in leaves of Pisum sativum after exposure to supplementary UV-B. — Plant Cell Physiol. 33: 1015–1023, 1992.
Sullivan, J.H., Teramura, A.H.: Field study of the interaction between solar ultraviolet-B radiation and drought on photosynthesis and growth in soybean. — Plant Physiol. 92: 141–146, 1990.
Teramura, A.H., Sullivan, J.H.: Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. — Photosynth. Res. 39: 463–473, 1994.
Tevini, M., Teramura, A.H.: UV-B effects on terrestrial plants. — Photochem. Photobiol. 50: 479–487, 1989.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the CSIR, New Delhi, India in the form of JRF to Rajiv Dwivedi.
Rights and permissions
About this article
Cite this article
Prasad, S.M., Dwivedi, R. & Zeeshan, M. Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica 43, 177–185 (2005). https://doi.org/10.1007/s11099-005-0031-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11099-005-0031-0