Abstract
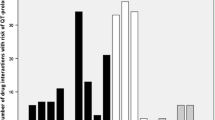
Background QT prolongation and associated arrhythmias, torsades de pointes (TdP), are considerable negative outcomes of many antipsychotic and antidepressant agents frequently used by psychiatric patients. Objective To identify the prevalence, levels, and predictors of QT prolonging drug–drug interactions (QT-DDIs), and AZCERT (Arizona Center for Education and Research on Therapeutics) classification of drugs involved in QT-DDIs. Setting Psychiatry wards of three major tertiary care hospitals of Khyber-Pakhtunkhwa, Pakistan. Method This was a multicenter cross-sectional study. Micromedex DrugReax was used for identification of QT-DDIs. TdP risks were identified by the AZCERT classification. Multivariate logistic regression analysis was performed to identify predictors of QT-DDIs. Main outcome measure Prevalence of QT-DDIs (overall, age-wise and gender-wise) and their levels of severity and documentation; AZCERT classes of drugs involved in QT-DDIs; and odds ratios for predictors of QT-DDIs. Results Of 600 patients, 58.5% were female. Median age was 25 years (IQR = 20–35). Overall 51.7% patients had QT-DDIs. Of total 698 identified QT-DDIs, most were of major-severity (98.4%) and fair-documentation (93.7%). According to the AZCERT classification, 36.4% of the interacting drugs were included in list-1 (known risk of TdP), 26.9% in list-2 (possible risk of TdP) and 27.5% in list-3 (conditional risk of TdP). Drugs commonly involved in QT-DDI were olanzapine (n = 146), haloperidol (138), escitalopram (122), risperidone (91), zuclopenthixol (87), quetiapine (n80) and fluoxetine (74). In multivariate logistic regression analysis, QT-DDIs were significantly associated with 6–7 prescribed medications (p = 0.04) and >7 medications (p = 0.03). Similarly, there was significant association of occurrence of QT-DDIs with 2–3 QT drugs (p < 0.001) and >3 QT drugs (p < 0.001). Conclusion A considerable number of patients are exposed to QT-DDIs in psychiatry. There is a need to implement protocol for monitoring the outcomes of QT-DDIs.
Similar content being viewed by others
References
Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7.
Armahizer MJ, Seybert AL, Smithburger PL, Kane-Gill SL. Drug–drug interactions contributing to QT prolongation in cardiac intensive care units. J Crit Care. 2013;28:243–9.
Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–34.
Roden DM, Hoffman BF. Action potential prolongation and induction of abnormal automaticity by low quinidine concentrations in canine Purkinje fibers. Relationship to potassium and cycle length. Circ Res. 1985;56:857–67.
Sicouri S, Antzelevitch C. Drug-induced afterdepolarizations and triggered activity occur in a discrete subpopulation of ventricular muscle cells (M cells) in the canine heart: quinidine and digitalis. J Cardiovasc Electrophysiol. 1993;4:48–58.
Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22.
Akar FG, Yan GX, Antzelevitch C, Rosenbaum DS. Unique topographical distribution of M cells underlies reentrant mechanism of torsade de pointes in the long-QT syndrome. Circulation. 2002;105:1247–53.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–47.
Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–74.
Vandael E, Marynissen T, Reyntens J, Spriet I, Vandenberghe J, Willems R, et al. Frequency of use of QT-interval prolonging drugs in psychiatry in Belgium. Int J Clin Pharm. 2014;36:757–65.
Wood AJ, Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22.
Darpo B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl. 2001;3:K70–80.
R. Girardin F, Gex-Fabry M, Berney P, Shah D, Gaspoz J-M, Dayer P. Drug-induced long QT in adult psychiatric inpatients: the 5-year cross-sectional ECG screening outcome in psychiatry study. Am J Psychiatr. 2013;170:1468–76.
Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269:1532–6.
Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96:1698–703.
Ng TM, Olsen KM, McCartan MA, Puumala SE, Speidel KM, Miller MA, et al. Drug-induced QTc-interval prolongation in the intensive care unit: incidence and predictors. J Pharm Pract. 2010;23:19–24.
Enger C, Cali C, Walker AM. Serious ventricular arrhythmias among users of cisapride and other QT-prolonging agents in the United States. Pharmacoepidemiol Drug Saf. 2002;11:477–86.
Honig PK, Wortham DC, Zamani K, Conner DP, Mullin JC, Cantilena LR. Terfenadine-ketoconazole interaction. Pharmacokinetic and electrocardiographic consequences. JAMA. 1993;269:1513–8.
Radosevic N, Gantumur M, Vlahovic-Palcevski V. Potentially inappropriate prescribing to hospitalised patients. Pharmacoepidemiol Drug Saf. 2008;17:733–7.
Gorard DA. Escalating polypharmacy. QJM. 2006;99:797–800.
Smithburger PL, Seybert AL, Armahizer MJ, Kane-Gill SL. QT prolongation in the intensive care unit: commonly used medications and the impact of drug–drug interactions. Expert Opin Drug Saf. 2010;9:699–712.
Magro L, Moretti U, Leone R. Epidemiology and characteristics of adverse drug reactions caused by drug–drug interactions. Expert Opin Drug Saf. 2012;11:83–94.
Random number generator. http://stattrek.com/statistics/random-number-generator.aspx. Accessed 5 Dec 2016.
Micromedex DrugReax. https://www.micromedexsolutions.com/home/dispatch/ssl/true. Accessed 10 Jan 2017.
Patel RI, Beckett RD. Evaluation of resources for analyzing drug interactions. J Med Libr Assoc. 2016;104:290.
Kheshti R, Aalipour M, Namazi S. A comparison of five common drug–drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract. 2016;5:257.
Reis AMM, Cassiani SHDB. Evaluation of three brands of drug interaction software for use in intensive care units. Pharm World Sci. 2010;32:822–8.
The Arizona center for education and research on therapeutics (AZCERT). https://crediblemeds.org/healthcare-providers/. Accessed 10 Dec 2016.
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2017. http://www.whocc.no/atc_ddd_index. Accessed 2 Feb 2017.
Moreno-Gutierrez PA, Gaviria-Mendoza A, Canon MM, Machado-Alba JE. High prevalence of risk factors in elderly patients using drugs associated with acquired torsades de pointes chronically in Colombia. Br J Clin Pharmacol. 2016;82:504–11.
Curtis LH, Ostbye T, Sendersky V, Hutchison S, Lapointe NMA, Al-Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114:135–41.
Mirza I, Jenkins R. Risk factors, prevalence, and treatment of anxiety and depressive disorders in Pakistan: systematic review. BMJ. 2004;328:794.
Ismail M, Iqbal Z, Khattak MB, Javaid A, Khan MI, Khan TM, et al. Potential drug–drug interactions in psychiatric ward of a tertiary care hospital: prevalence, levels and association with risk factors. Trop J Pharm Res. 2012;11:289–96.
Farooq S, Akhtar J, Nazar Z, Khan SA. Sociodemographic and clinical characteristics of iv drug users presenting to a tertiary care treatment centre. J Postgrad Med Inst (Peshawar-Pakistan) 2011;20:3–7.
Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–17.
Ferdinand RF, Verhulst FC, Wiznitzer M. Continuity and change of self-reported problem behaviors from adolescence into young adulthood. J Am Acad Child Adolesc Psychiatry. 1995;34:680–90.
Sala M, Vicentini A, Brambilla P, Montomoli C, Jogia JR, Caverzasi E, et al. QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry. 2005;4:1.
Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54:1–13.
Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–35.
Yuksel FV, Tuzer V, Goka E. Escitalopram intoxication. Eur Psychiatry. 2005;20:82.
Nykamp DL, Blackmon CL, Schmidt PE, Roberson AG. QTc prolongation associated with combination therapy of levofloxacin, imipramine, and fluoxetine. Ann Pharmacother. 2005;39:543–6.
Haueis P, Greil W, Huber M, Grohmann R, Kullak-Ublick GA, Russmann S. Evaluation of drug interactions in a large sample of psychiatric inpatients: a data interface for mass analysis with clinical decision support software. Clin Pharmacol Ther. 2011;90:588–96.
Abarca J, Malone DC, Armstrong EP, Grizzle AJ, Hansten PD, Van Bergen RC, et al. Concordance of severity ratings provided in four drug interaction compendia. JAPhA. 2003;44:136–41.
Vitry AI. Comparative assessment of four drug interaction compendia. Br J Clin Pharmacol. 2007;63:709–14.
Wong CM, Ko Y, Chan A. Clinically significant drug–drug interactions between oral anticancer agents and nonanticancer agents: profiling and comparison of two drug compendia. Ann Pharmacother. 2008;42:1737–48.
Roblek T, Vaupotic T, Mrhar A, Lainscak M. Drug–drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol. 2015;71:131–42.
Acknowledgements
We are very grateful to hospital administration, physicians and all other staff for their cooperation in this study.
Funding
No specific funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None to declare.
Rights and permissions
About this article
Cite this article
Khan, Q., Ismail, M., Haider, I. et al. Prevalence of QT interval prolonging drug–drug interactions (QT-DDIs) in psychiatry wards of tertiary care hospitals in Pakistan: a multicenter cross-sectional study. Int J Clin Pharm 39, 1256–1264 (2017). https://doi.org/10.1007/s11096-017-0532-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-017-0532-5