Abstract
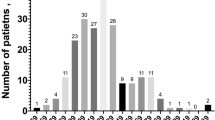
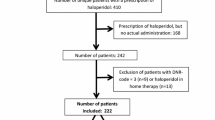
Background More than 170 drugs are linked with QTc-prolongation, which in extreme cases can lead to Torsade de Pointes. Monitoring of this potential side effect is an important challenge in clinical practice. Objective To investigate the risk of QTc-prolongation in hospital patients who started a QTc-prolonging drug, and to develop a risk score to identify patients at high/low risk for QTc-prolongation. Setting University Hospitals Leuven, Belgium. Method All patients starting with haloperidol or a QTc-prolonging antibiotic/antimycotic were eligible for this observational study. Twelve-lead electrocardiograms were recorded at baseline and follow-up (steady state). Demographic, medical and drug data were collected. The obtained data were used to calculate the performance characteristics of a preliminary risk score (RISQ-PATH score), based on a systematic review of risk factors. ROC analysis determined a score of <10 points as a low risk for QTc-prolongation. Main outcome measure QTc-interval in a baseline and follow-up electrocardiogram. Results 178 patients (46.6% female; mean age 69 ± 14 years) were included (levofloxacin: N = 80; haloperidol: N = 41; fluconazole: N = 41). Overall, no significant difference between the mean QTc-values at baseline (425.7 ± 31.7 ms) and follow-up (428.0 ± 30.7 ms) was found (p = 0.328). However, 26 patients (14.6%) did develop a prolonged QTc-interval (≥450(♂)/470(♀) ms) of whom 25 with a RISQ-PATH score ≥10. This score had a sensitivity of 96.2% (95% CI 78.4–99.8%) and a negative predictive value of 98.0% (95% CI 88.2–99.9%). Conclusion This RISQ-PATH score is able to rule out low-risk patients with a negative predictive value of 98.0% and is promising to exclude patients from further follow-up when starting QTc-prolonging drugs.
Clinicaltrials.gov Registration Number: NCT02068170.


Similar content being viewed by others
References
Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22.
Malik M, Hnatkova K, Kowalski D, Keirns JJ, van Gelderen EM. Importance of subject-specific QT/RR curvatures in the design of individual heart rate corrections of the QT interval. J Electrocardiol. 2012;45:571–81.
Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc. 2016;. doi:10.1161/JAHA.116.003264.
CPMP/986/96. The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. London: Committee for Proprietary Medicinal Products; 1997.
Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17:333–6.
Nielsen JB, Graff C, Rasmussen PV, Pietersen A, Lind B, Olesen MS, et al. Risk prediction of cardiovascular death based on the QTc interval: evaluating age and gender differences in a large primary care population. Eur Heart J. 2014;35:1335–44.
Pickham D, Helfenbein E, Shinn JA, Chan G, Funk M, Weinacker A, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in Practice (QTIP) Study. Crit Care Med. 2012;40:394–9.
U.S. Department of Health and Human Services. International conference on harmonisation (ICH). Guidance for Industry: E14 Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. 2005. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed 6 Feb 2017.
Woosley RL, Romero KA. QTdrugs List, December 2015, AZCERT, Inc. 1822 Innovation Park Dr., Oro Valley, AZ 85755. www.crediblemeds.org. Accessed 9 Nov 2016.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–47.
Vandael E, Vandenberk B, Vandenberghe J, Spriet I, Willems R, Foulon V. Risk management of QTc-prolongation in patients receiving haloperidol: an observational study in a university hospital in Belgium. Int J Clin Pharm. 2016;38:310–20.
Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88:315–25.
Tisdale JE, Jaynes HA, Kingery JR, Mourad NA, Trujillo TN, Overholser BR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–87.
Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm. 2017;39:16–25. doi:10.1007/s11096-016-0414-2.
WHO Collaborating Centre for Drug Statistics Methodology: ATC/DDD Index (online), 2016. http://www.whocc.no/atc_ddd_index. Accessed 14 Apr 2016.
Flockhart DA. Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine. 2007. http://medicine.iupui.edu/clinpharm/ddis/main-table. Accessed 14 Apr 2016.
Cheung D, Wolfe B, Wald H, Cumbler E. Unsafe use of intravenous haloperidol: evaluation of recommendation-concordant care in hospitalized elderly adults. J Am Geriatr Soc. 2013;61:160–1.
Jardin CG, Putney D, Michaud S. Assessment of drug-induced torsade de pointes risk for hospitalized high-risk patients receiving QT-prolonging agents. Ann Pharmacother. 2014;48:196–202.
Warnier M, Rutten F, Souverein P, Hoes A, de Boer A, De Bruin M. Are ECG monitoring recommendations before prescription of QT prolonging drugs applied in daily practice? The example of haloperidol. Pharmacoepidemiol Drug Saf. 2014;23:228–9.
Blom MT, Bardai A, van Munster BC, Nieuwland MI, de Jong H, van Hoeijen DA, et al. Differential changes in QTc duration during in-hospital haloperidol use. PLoS ONE. 2011;6:e23728.
van Noord C, Eijgelsheim M, Stricker BH. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16–23.
Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. Herg K + channels: structure, function, and clinical significance. Physiol Rev. 2012;92:1393–478.
Schwartz PJ, Woosley RL. Predicting the unpredictable: drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67:1639–50.
Acknowledgements
We want to thank the University Hospitals Leuven, especially the treating physicians, nursing staff and IT service.
Funding
Ph.D.-student EV is supported by funding of the Belgian government agency for Innovation by Science and Technology (IWT). RW is supported as a clinical researcher by the Fund for Scientific Research Flanders. IS is supported by the Clinical Research Fund of the University Hospitals Leuven.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vandael, E., Vandenberk, B., Vandenberghe, J. et al. Development of a risk score for QTc-prolongation: the RISQ-PATH study. Int J Clin Pharm 39, 424–432 (2017). https://doi.org/10.1007/s11096-017-0446-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-017-0446-2