Abstract
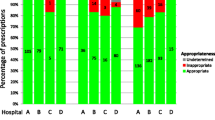
Background Antimicrobial guidelines aim to optimise treatment and minimise development of resistance. Care bundles support the implementation of local guidelines. Objective Pharmacist identification of where in the prescribing, monitoring and documentation process, the quality of antimicrobial management in a High Dependency Unit of a large teaching hospital could be improved and design of antimicrobial care bundles for initiation and de-escalation of therapy to standardise care and improve practice. Setting This study was conducted in a 10-bed, mixed medical-surgical HDU in a large Scottish (UK) teaching hospital. Methods Quality indicators (n = 30) were agreed through multidisciplinary team review with reference to the evidence base, national strategy and local policy. Adherence to these quality indicators was measured before and after the pharmacist’s contributions. Areas of non-adherence to quality indicators were used to design the care bundles. Main outcome measure Adherence to the quality indicators before and after the pharmacist’s action. Categorisation of pharmaceutical care issues (‘check’, ‘change in drug therapy’ and ‘change in drug therapy process’) were quantified. Results From 134 prescriptions, the pharmacist undertook 1,447 actions to ensure adherence to the 30 indicators. Adherence was very good (85.3 % CI 83.5, 87.1), but would have been unsatisfactory (53.5 % CI 50.9, 56.1) without the pharmacist’s action (p < 0.001). Change in drug therapy process or change in drug therapy initiated by the pharmacist accounted for 31.9 % (CI 29.5, 34.3) of adherence. Non-adherence was related to documentation of past allergic reactions, bacteriological specimen results, indication and length of course of treatment (both at initiation and de-escalation). Care bundles were designed to target areas of non-adherence to local guidelines. Conclusion The pharmacist made a significant contribution to improving adherence to evidence based antimicrobial prescribing quality indicators agreed by the multidisciplinary team. Prompts have been identified from the pharmaceutical care process and applied in the design of two antimicrobial care bundles proposed to support adherence with antimicrobial prescribing policies and guidelines.


Similar content being viewed by others
References
Healthcare Associated Infection Task Force. The Scottish Management of Antimicrobial Resistance Action Plan (ScotMARAP). Edinburgh: Scottish Government; 2008. ISBN 978 0 7559 7039 1.
Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77.
Institute of healthcare improvement. What is a care bundle? In: Knowledge centre, Institute of healthcare improvement. http://www.ihi.org/knowledge/Pages/ImprovementStories/WhatIsaBundle.aspx (14 June 2012).
Scottish Patient Safety Programme. In: Programme, Scottish patient safety alliance. http://www.patientsafetyalliance.scot.nhs.uk/programme (14 June 2012).
Morris AC, Hay AW, Swann DG, Everingham K, McCulloch C, McNulty J, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med. 2011;39(10):2218–24.
Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67(9):746–9.
Dunn K, O’Reilly A, Silke B, Rogers T, Bergin C. Implementing a pharmacist-led sequential antimicrobial therapy strategy: a controlled before-and-after study. Int J Clin Pharm. 2011;33:208–14.
General Medical Council. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education—EQUIP study. In: Completed Research, GMC Research, 3 December 2009. http://www.gmc-uk.org/FINAL_Report_prevalence_and_causes_of_prescribing_errors.pdf_28935150.pdf (14 June 2012).
Scottish Medicines Consortium/Healthcare Associated Infection Taskforce. Antimicrobial prescribing policy and practice in Scotland: recommendations for good antimicrobial practice in acute hospitals. Edinburgh: Scottish Executive; 2005. ISBN 0 7559 2698.
Scottish Government, CEL 11. A revised framework for national surveillance of healthcare associated infection and the introduction or a new health efficiency and access to treatment (HEAT) target for clostridium difficile associated disease (CDAD) for NHS Scotland. Edinburgh: Scottish Government; 2009.
Zarb P, Amadeo B, Muller A, Drapier N, Vankerckhoven V, Davey P, et al. Identification of targets for improvement in antimicrobial prescribing: the web based ESAC Point Prevalence Survey 2009. J Antimicrob Chemother. 2011;66(2):443–9.
Pulcini C, Defres S, Aggarwal I, Nathwani D, Davey P. Design of a “day 3 bundle” to improve the reassessment of inpatient empirical antibiotic prescriptions. J Antimicrob Chemother. 2008;61(6):1384–8.
Cooke FJ, Holmes AH. The missing care bundle: antibiotic prescribing in hospitals. Int J Antimicrob Agents. 2007;30(1):25–9.
NHS Lothian University Hospitals Division. Antimicrobial prescribing guidelines in adults (version 1.3). Edinburgh: Area Drugs and Therapeutics Committee; 2009.
NHS Lothian Critical care directorate. Antimicrobial guideline. Edinburgh: Critical care directorate; 2009.
Skogly S. A study of patient pharmaceutical care needs assessment in chronic obstructive pulmonary disease; as an example of a multidisciplinary intervention to reduce hospital re-admission in long term conditions. Glasgow: University of Strathclyde/University of Tromso; 2009.
Grimshaw JM, Thomas RE, Maclennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):1–72.
Institute for Healthcare Improvement. Science of Improvement. In: Knowledge centre, Institute of healthcare improvement. http://www.ihi.org/knowledge/Pages/HowtoImprove/ScienceofImprovementFormingtheTeam.aspx (14 June 2012).
British National Formulary. 59th ed. London: BMJ Group/Pharmaceutical Press; 2010. ISBN 978 0 8536 9929 3.
Baxter K. Stockley’s drug interactions. 8th ed. London: Pharmaceutical Press; 2008. ISBN 978 0 8536 9754 1.
Ashley C, Currie A. The renal drug handbook. 3rd ed. Oxon: Radcliffe Publishing; 2009. ISBN 978 1 8461 9298 2.
Acknowledgments
We acknowledge the help of Clinical Pharmacists Claire Hannah and Laura Shaw and Drs Alasdair Hay, Christian Helgason, Karen MacSween, David Swann and Elizabeth Wilson for their advice regarding the quality indicators and care bundles.
Funding
Data has been generated as part of the routine work of the pharmacist. No external funding was received for this work.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coll, A., Kinnear, M. & Kinnear, A. Design of antimicrobial stewardship care bundles on the high dependency unit. Int J Clin Pharm 34, 845–854 (2012). https://doi.org/10.1007/s11096-012-9680-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-012-9680-9