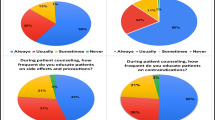
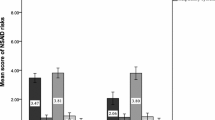
Abstract
Objectives To validate and pilot in Thailand a questionnaire to enable patients to identify and report symptoms perceived as potential ADRs from NSAIDs. To determine the questionnaire’s usefulness in enabling Thai out-patients to report potential ADRs. To determine the frequency with which symptoms patients reported were recorded by health professionals and the frequency of ADRs to these drugs reported to the APRM Centre. To assess whether patients reported symptoms from non COX-selective inhibitors and COX-2 selective NSAIDs with different frequencies. Setting Out-patient departments (OPD) of a University teaching hospital in North-East Thailand. Method A questionnaire which incorporated an extensive symptoms checklist, developed and validated in English, was translated, piloted and validated in Thai. This was distributed to patients receiving one of five NSAIDs. Causality assessment of the symptoms reported was undertaken by a pharmacist, using data on concomitant medicines and disease states from OPD records. Outcome measures Frequency and type of symptoms reported by patients, recording of these in OPD records, reports sent to APRM Centre. Results Piloting found that patients were able to understand the questionnaire, but were unaware of drug names. A response rate of 42% was obtained: 694 usable questionnaires were returned out of 1,654 distributed. Overall 73% of respondents reported at least one symptom perceived to be an ADR. Sixty percent of symptoms reported were classed as probably or possibly an ADR. Fewer symptoms per patient were reported by those taking COX-2 selective inhibitors (3.5) than those taking non-selective NSAIDs (5.5), although there were no differences in the frequency of GI symptoms reported between these two sub-classes, which may relate to other factors, such as age, previous GI problems and prescription of protective ulcer-healing therapy. Only 5% of symptoms were recorded in OPD records and reporting of ADRs to these drugs to the APRM Centre of the Thai FDA during the study was very limited. Conclusion Thai out-patients were willing and able to complete questionnaires regarding potential ADRs. The questionnaire could form part of routine out-patient monitoring, aiding identification of ADRs, and may help to increase ADR reporting in Thailand.
Similar content being viewed by others
References
Adverse Product Reaction Monitoring Center. Adverse Drug Reactions Reporting 2004. Bangkok; 2004.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–96.
Chaikoolvatana A, Chanakit T, Juengrakpong A. The evaluation of a recurrent adverse drug reaction prevention program in the North-East region of Thailand. J Med Assoc Thai. 2006;89(5):699–705.
Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63(2):148–56. doi:10.1111/j.1365-2125.2006.02746.x.
Egberts TCG, Smulders M, Koning FHP. Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. Br Med J. 1996;313:530–1.
Jarernsiripornkul N, Krska J, Capps PAG, et al. Patient reporting of potential adverse drug reactions: a methodological study. Br J Clin Pharmacol. 2002;53:318–25.
Brooks P. Use and benefits of non-steroidal anti-inflammatory drugs. Am J Med. 1998;104(3):9S–13S.
Gurwitz JH, Avorn J. The ambiguous relation between aging and adverse drug reactions. Ann Intern Med. 1991;150:841–5.
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. Br Med J. 2004;329:15–9.
Rostom A, Muir K, Dube C, et al. Gastrointestinal safety of cyclooxygenase-2 inhibitors: a cochrane collaboration systematic review. Clin Gastroenterol Hepatol. 2007;5:818–28.
Jarernsiripornkul N, et al. English version of questionnaire. http://www.blackwellpublishing.com/products/journals/suppmat/BCP/BCP1547/BCP1547_fsms1.pdf.
Mitchell AS, Henry DA, Hennrikus D, et al. Adverse drug reactions: can consumers provide early warning? Pharmacoepidemiol Drug Saf. 1994;3:257–64.
Agbabiaki TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions. Drug Saf. 2008;31:21–137.
Lewis SC, Langman MJS, Laporte J-R, et al. Dose-response relationships between individual non-aspirin non-steroidal anti-inflammatory drugs (NANSAIDs) and serious upper gastrointestinal bleeding: a meta-analysis based on individual patient data. Br J Clin Pharmacol. 2002;54:320–6.
Mitchell AS, Henry DA, Fisher S, et al. Patients as a direct source of information on adverse drug reactions. Br Med J. 1988;297:891–3.
Van den Bemt PMLA, Egberts ACG, Lenderink AW, et al. Pharmacoepidemiology and prescription: adverse drug events in hospitalized patients. A comparison of doctors, nurses and patients as sources of reports. Eur J Clin Pharmacol. 1999;55:155–8.
Cox AR. Patient reporting of adverse drug reactions. PharmacoVigilance Rev. 2009;3:18–21.
Acknowledgements
We would like to thank the pharmacists, doctors and other staff working at Srinagarind Hospital, Khon Kaen University. Special thanks are extended to all respondents and Adverse Product Reaction Monitoring Center of the Thai FDA for supplying valuable data.
Funding
This study was funded by a grant from Khon Kaen University.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jarernsiripornkul, N., Chaisrisawadsuk, S., Chaiyakum, A. et al. Patient self-reporting of potential adverse drug reactions to non-steroidal anti-inflammatory drugs in Thailand. Pharm World Sci 31, 559–564 (2009). https://doi.org/10.1007/s11096-009-9310-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-009-9310-3