Abstract
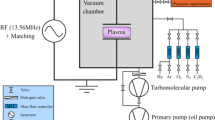
Remarkable properties of plasma polymer films are greatly dependent not only on the chemical structure of precursor but also on the reactor design and the deposition conditions. In many industrial applications it is a challenge to control the plasma polymer structure. In this paper we investigate the chemical transformation of various aromatic compounds, such as activation and fragmentation of substituent-part, aromatic ring opening, during plasma polymerization process. Polymerized films are deposited in a low-frequency capacitively coupled plasma-enhanced chemical vapour deposition reactor, working at low pressure. The chemical composition of plasma-polymerized films is elucidated by Fourier-transform infrared spectroscopy, solid-state carbon-13 nuclear magnetic resonance spectroscopy, and X-ray photoelectron spectroscopy. Based on spectroscopic measurements, the intermediary reactions during film growth may be presumed.












Similar content being viewed by others
References
Asandulesa M, Topala I, Pohoata V, Dumitrascu N (2010) Influence of operational parameters on plasma polymerization process at atmospheric pressure. J Appl Phys 108:093310–093316
d’Agostino R, Cramarossa F, Fracassi F (1990) Plasma polymerization of fluorocarbons. Academic Press, New York
Albaugh J, O’Sullivan C, O’Neill L (2008) Controlling deposition rates in an atmospheric pressure plasma system. Surf Coat Technol 203:844–847
Arefi F, Andre V, Montazer-Rahmati P, Amouroux J (1992) Plasma polymerization and surface treatment of polymers. Pure Appl Chem 64:715–723
Asandulesa M, Topala I, Pohoata V, Legrand YM, Dobromir M, Totolin M, Dumitrascu N (2013) Chemically polymerization mechanism of aromatic compounds under atmospheric pressure plasma conditions. Plasma Process Polym 10:469–480
Forch R, Chifen AN, Bousquet A, Khor HL, Jungblut M, Chu L-Q, Zhang Z, Osey-Mensah I, Sinner E-K, Knoll W (2007) Recent and expected roles of plasma-polymerized films for biomedical applications. Chem Vap Depos 13:280–294
Yasuda H, Hirotsu T (1978) Critical evaluation of conditions of plasma polymerization. J Polym Sci Polym Chem 16:743–759
Yasuda H (1985) Plasma polymerization. Academic Press, New York
Hegemann D, Hossain MM, Korner E, Balazs DJ (2007) Macroscopic description of plasma polymerization. Plasma Process Polym 4:229–238
Hegemann D, Korner E, Guimond S (2010) Reply to: ‘‘testing the hypothesis: comments on plasma polymerization of acrylic acid revisited’’. Plasma Process Polym 7:371–375
Stille JK, Sung RL, Van der Kooi J (1965) The reaction of benzene in a radiofrequency glow discharge. J Org Chem 30:3116–3119
Kaplan S, Dilks A (1983) The structure of plasma-polymerized toluene: a solid-state 13C-NMR study of isotopically labeled polymers. J Polym Sci Pol Chem 21:1819–1829
Friedrich J (2011) Mechanisms of plasma polymerization—reviewed from a chemical point of view. Plasma Process Polym 8:783–802
Socrates G (2004) Infrared and raman characteristic group frequencies. Wiley, Baffins Lane, Chichester
Yang JC, Jablonsky MJ, Mays JW (2002) NMR and FT[hyphen]IR studies of sulfonated styrene[hyphen]based homopolymers and copolymers. Polymer 43:5125–5132
Tolstorozhev G, Skornyakov I, Bel’kov M, Shadyro O, Brinkevich S, Samovich S (2012) IR spectra of benzaldehyde and its derivatives in different aggregate states. Opt Spectrosc 113:179–183
Reichenbächer M, Popp J (2012) Challenges in molecular structure determination. Springer, Berlin
Mannion JJ, Wang TS (1964) An infrared study of the CH2Cl group in benzyl chloride and derivatives. Spectrochim Acta 20:45–49
Spectral Database for Organic Compounds (SDBS), National Institute of Advanced Industrial Science and Technology (AIST), Japan. http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi. Accessed August 2013
Merche D, Poleunis C, Bertrand P, Sferrazza M, Reniers F (2009) Synthesis of polystyrene thin films by means of an atmospheric-pressure plasma torch and a dielectric barrier discharge. IEEE Trans Plasma Sci 37:951–960
NIST X-Ray Photoelectron Spectroscopy Database, NIST Standard Reference Database 20, Version 3.5. http://srdata.nist.gov/xps/. Accessed August 2013
Zhang C, Hu J, Cong J, Zhao Y, Shen W, Toyoda H, Nagatsu M, Meng Y (2011) Pulsed plasma-polymerized alkaline anion-exchange membranes for potential application in direct alcohol fuel cells. J Power Sources 196:1–8
Luo HL, Sheng J, Wan YZ (2007) Plasma polymerization of styrene with carbon dioxide under glow discharge conditions. Appl Surf Sci 253:5203–5207
Gadgil JM, Rajan CR, Ponrathnam S, Rajamohanan PR, Ganapathy S (1991) Acrolein-acrylic acid copolymers: a solid-state 13C-NMR study of free aldehyde groups. J Polym Sci Pol Chem 29:1077–1081
Joseph R, Ford WT, Zhang S, Tsyurupa MP, Pastukhov AV, Davankov VA (1997) Solid-state 13C-NMR analysis of hypercrosslinked polystyrene. J Polym Sci Pol Chem 35:695–701
Wu RR, Kao HM, Chiang JC, Woo EM (2002) Solid-state NMR studies on phase behavior and motional mobility in binary blends of polystyrene and poly(cyclohexyl metacrylate). Polymer 43:171–176
Law RV, Sherrington DC, Snape CE (1996) Solid-state 13C MAS NMR studies of hyper-cross-linked polystyrene resins. Macromolecules 29:6284–6293
Merche D, Vandencasteele N, Reniers F (2012) Atmospheric plasmas for thin film deposition: a critical review. Thin Solid Films 52:4219–4236
Bothe M, Schmidt-Naake G (2004) Nitroxide-mediated radical polymerization with bisaminooxy compounds as initiators—controlled biradical polymerization. Macromol Chem Phys 205:208–216
Sanderson RT (1971) Chemical bonds and bond energy. Academic Press, London
Acknowledgments
Philippe Gaveau, Marius Dobromir and Romain Coustel are gratefully acknowledged for their valuable help in 13C-NMR, XPS and SEM analyses, respectively. This work was supported by European Union’s Seventh Framework Programme (Grant Agreement No. 264115-STREAM, call FP7-REGPOT-2010-1) and Romanian Space Agency (ROSA), project STAR CDI ID 349/2014-2016.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paper dedicated to the 65th anniversary of “Petru Poni” Institute of Macromolecular Chemistry of Romanian Academy, Iasi, Romania.
Rights and permissions
About this article
Cite this article
Asandulesa, M., Topala, I., Legrand, YM. et al. Chemical Investigation on Various Aromatic Compounds Polymerization in Low Pressure Helium Plasma. Plasma Chem Plasma Process 34, 1219–1232 (2014). https://doi.org/10.1007/s11090-014-9555-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-014-9555-z