Abstract
Wheat grain color does not only affect the brightness of flour but also seed dormancy and pre-harvest sprouting (PHS) tolerance. The transcription factor Tamyb10 is an important candidate for R-1 gene, and the expression of its homologs determines wheat seed coat color. In the present study, the allelic variations of Tamyb10 were explored in a set of Chinese bread wheat varieties and advanced lines with different PHS tolerance, and a sequenced-tagged site (STS) marker for Tamyb10-D1 gene was developed, designated as Tamyb10D, which could be used as an efficient and reliable marker to evaluate the depth dormancy of wheat seeds. Using the marker Tamyb10D, 1629- and 1178-bp PCR fragments were amplified from the tolerant varieties, whereas a 1178-bp fragment was from the susceptible ones. Of the Chinese bread wheat varieties and advanced lines, 103 were used to validate the relationship between the polymorphic fragments of Tamyb10D and PHS tolerance. Statistical analysis indicated that Tamyb10D was significantly (P < 0.001) associated with depth of seed dormancy in these germplasms. To further confirm the association between allelic variants of Tamyb10-D1 and PHS tolerance, 200 recombinant inbred lines (RILs) from the cross between Zhongyou 9507 (1178-bp fragment) and Yangxiaomai (1178- and 1629-bp fragments) were genotyped using the marker Tamyb10D. General linear model analysis indicated that variation in Tamyb10-D1 had a significant (P < 0.001) association with the germination index (GI) values, explaining 13.7, 4.7, and 9.8 % of the phenotypic variation in GI in Shijiazhuang, Beijing, and the averaged data from those environments, respectively. In addition, among the 103 wheat varieties, 8 Tamyb10 genotypes (Tamybl0-A1, Tamybl0-B1, and Tamyb10-D1 loci) were detected, namely, aaa, aab, aba, abb, baa, bab, bba, and bbb, and these were significantly associated with GI value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pre-harvest sprouting (PHS) results in loss of grain weight and reduction in the end-use quality of kernels in cereals, especially in wheat. It is believed that PHS tolerance of wheat is predominantly attributed to seed dormancy (Bailey et al. 1999; Flintham 2000; Li et al. 2004; Gubler et al. 2005; Tan et al. 2006; Yang et al. 2007; Sun et al. 2012; Yang et al. 2014). Improvement of tolerance to PHS in wheat is a major breeding objective in China and many other countries such as Japan, Australia, Canada, and USA. Therefore, an understanding of genetic control of seed dormancy or PHS tolerance and development of functional markers are very important for marker-assisted breeding targeting for improvement of PHS tolerance in wheat.
Seed dormancy can be divided into embryo-imposed and coat-imposed dormancy. Many genes involved in seed dormancy are known to be involved in abscisic acid (ABA) synthesis and ABA signal transduction (Gubler et al. 2005). The viviparous (Vp-1) gene is an important regulator of late embryogenesis in maize and a regulator of late embryo development in bread wheat (McCarty et al. 1991). AtABI3 in Arabidopsis is an orthologous gene of ZmVp-1 in maize and OsVp-1 in rice (Koornneef et al. 1989; McCarty et al. 1991; Hattori et al. 1994). Furthermore, the AtLEC2, AtDOG1, AtHUB1, and KYP/SUVH4 genes are also important for controlling seed dormancy (Stone et al. 2001; Bentsink et al. 2006; Liu et al. 2007; Zheng et al. 2012). In wheat, TaSdr genes were cloned and proved to be associated with tolerance to PHS in bread wheat (Zhang et al. 2014). Moreover, the TaVp-1 genes involved in ABA signal transduction are also important seed dormancy-related transcription factors, and a positive correlation was present between seed dormancy and embryo sensitivity to ABA (Nakamura and Toyama 2001; McKibbin et al. 2002). Six alleles of TaVp-1B were identified, designated as TaVp-1Ba, TaVp-1Bb, TaVp-1Bc, TaVp-1Bd, TaVp-1Be, and TaVp-1Bf, respectively (Yang et al. 2007; Xia et al. 2008; Yang et al. 2009; Chang et al. 2010). Based on the allelic variation, a STS marker Vp1B3 associated with seed dormancy was developed; variations with TaVp-1Bb and TaVp-1Bc were associated with higher PHS tolerance (Yang et al. 2007). For TaVp-1A, rich allelic variations were detected (Chang et al. 2011; Sun et al. 2012; Yang et al. 2014) and another STS marker Vp1A3 for PHS tolerance was developed; the variations with the allele combinations TaVp-1Agm/TaVp-1Bb, TaVp-1Agm/TaVp-1Ba, TaVp-1Aim/TaVp-1Bb, and TaVp-1Aam/TaVp-1Bb showed higher PHS resistance (Yang et al. 2014).
The dormancy of lighter colored seed was weaker than that of darker colored seed in Arabidopsis (Debeaujon et al. 2000), indicating the association of grain dormancy with grain color. Wheat grain color is controlled by R-1 genes located in the distal region of the long arms of wheat chromosomes 3A, 3B, and 3D (R-A1, R-B1, and R-D1, respectively). The recessive alleles for white grains have been denoted R-A1a, R-B1a, and R-D1a, while the dominant alleles for red grains, R-A1b, R-B1b, and R-D1b, respectively. For dominant alleles R-A1b, R-B1b, and R-D1b, any one is sufficient to result in red grains, and grain’s redness increases in a gene dosage-dependent manner (McIntosh et al. 2010). The R-1 genes affect the sensitivity of embryos to ABA and the development of grain dormancy. It was proposed that one of the Myb-type genes of Arabidopsis, AtMYB2, might be involved in ABA signal transduction (Abe et al. 2003).
The pigment of red grain color is composed of anthocyan in anidin and catechin, which are synthesized by the enzymes chalcone synthase (CHS), chalcone flavanone isomerase (CHI), flavanone3-hydroxylase (F3H), and dihydroflavonol-4-reductase (DFR) in the flavonoid synthesis pathway (Holton and Cornish 1995; Chopra et al. 1996; Mol et al. 1998). Anthocyanins, phlobaphenes, flavonols, and proanthocyanidins (PAs) are all synthesized through the same early flavonoid biosynthetic pathway branched out into the individual pathway. CHS, CHI, F3H, and DFR are expressed mainly in the immature red grains and are almost totally suppressed in white grains (Himi and Noda 2005). In addition, several regulatory proteins involved in flavonoid biosynthesis have been reported in various species, such as maize, petunia, snapdragon, and Arabidopsis (Winkel-Shirley 2001; Mol et al. 1998). Two types of transcription factors grouped as the R/B family (basic helix-loop-helix (bHLH)-type) and the C1/Pl family (Myb-type) were able to upregulate all the structural genes required for the production of anthocyanin. Myb/c1 are transcriptional activators of flavonoid synthesis genes (Himi and Noda 2005), approximately 30 cM proximal to the Vp1 locus, consistent with observed linkage between grain dormancy and red grain (Groos et al. 2002; Himi and Noda 2004). Vp-1 is a key element that plays an important role in the seed maturation processes, such as seed dormancy and seed desiccation (McCarty et al. 1991; Giraudat et al. 1992). It was reported that Vp1/ABA/GA coordinated the control of grain color and PHS via Myb-dependent and Myb-independent pathways (Xia et al. 2009). Mutations in Vp-1 inhibit anthocyanin in synthesis (Robertson 1955; McCarty et al. 1989). Vp-1 interacts with the Sph cis-element in the promoter region of the Myb/c1 gene and regulates its expression (Hattori et al. 1992; Carson et al. 1997).
Hvmyb10 was also a key factor of grain dormancy in barley (Himi et al. 2012). Tamyb10- A1, Tamyb10-B1, and Tamyb10-D1 genes, located on chromosomes 3A, 3B, and 3D in wheat, respectively, encode R2R3-type MYB domain proteins, similar to TT2 of Arabidopsis that controls PA synthesis and induces the expression of flavonoid biosynthetic genes such as CHS, CHI, F3H, and DFR, which are also essential for the synthesis of both anthocyanins and proanthocyanidins (Himi et al. 2011). In addition, Tamyb10 was demonstrated to activate anthocyanin biosynthesis genes with synergy of the bHLH-type protein through a transient assay, and it is likely to be a strong candidate for the R-1 gene of wheat, which regulates wheat grain color (Himi et al. 2011); the red-grained wheat varieties are usually more tolerant to PHS than white-grained wheat varieties (Flintham 2000; Warner et al. 2000; Himi et al. 2002). However, not all the red-grained wheat varieties are higher PHS resistant than white grained, and Tamyb10-A1 also expressed in white-grained EMS-AUS in immature grain (DPA 5) (Himi et al. 2011), indicating that expression of Tamyb10 may play an important role in interacting with Vp-1 in PHS tolerance mechanism in white-grained wheat. In our previous study, some landraces, such as Suiningtuotuomai (average GI = 0.10) and Waitoubai (average GI = 0.07), had the strong PHS resistance, but they did not carry any of the PHS-resistant allele combinations of TaVp-1Agm/TaVp-1Bb, TaVp-1Agm/TaVp-1Ba, TaVp-1Aim/TaVp-1Bb, and TaVp-1Aam/TaVp-1Bb (Yang et al. 2014), which might lay in the fact that there were still other factors affecting PHS except for Vp-1 gene.
The objectives of the present study were to identify the allelic variations at Tamyb10 locus among Chinese wheat varieties and advanced lines with different level of PHS tolerance and develop efficient markers for marker-assisted breeding. Furthermore, the identification of these new Tamyb10 resources could also contribute to our understanding of the mechanisms underlying seed dormancy or PHS tolerance in bread wheat.
Materials and methods
Plant materials
Ten bread wheat varieties were used for PCR amplification of Tamyb10-A1, Tamyb10-B1, and Tamyb10-D1. There were five PHS-resistant varieties, Xiaobaiyuhua, Yumai 18, Yangxiaomai, Xiaoyuhua, and Xiaoye 6, with the germination index (GI) values of 0.04, 0.07, 0.08, 0.10, and 0.14, respectively, and five PHS-susceptible varieties, Zhou 8425B, Jimai 19, Jing 411, Hengshui 7228, and Zhongyou 9507, with the GI values of 0.56, 0.58, 0.64, 0.68, and 0.71, respectively.
In total, 103 Chinese varieties and advanced lines, with different PHS resistance from the China Autumn-sown Wheat Region (CAWR), representing more than 85 % of wheat production areas in China, were used for association study as presented in Table 1. Among these, 18 varieties had a GI less than 0.15 and 85 between 0.15 and 0.71. The GI was determined based on the average data across two cropping seasons at two locations, Anyang in Hennan Province and Beijing (Table 1) (Yang et al. 2014). Moreover, 200 recombinant inbred lines (RILs), derived from the Yangxiaomai/Zhongyou 9507 cross, were used to confirm relationship between the allelic variations in Tamyb10-D1 and PHS tolerance; Yangxiaomai is a Chinese landrace and had a low GI value (0.08), whereas Zhongyou 9507 had a high GI value (0.71).
Primer design
Nine gene-specific primers, Tamyb10-AF1/R1, Tamyb10-AF2/R2, Tamyb10-AF3/R3, Tamyb10-BF1/R1, Tamyb10-BF2/R2, Tamyb10-BF3/R3, Tamyb10-DF1/R1, Tamyb10-DF1/R2, and Tamyb10-DF3/R3, were used to amplify the Tamyb10-A1, Tamyb10-B1, and Tamyb10-D1 genes, respectively (Table 2). The other primer sets Myb10-A1, Myb10-A1, Myb10-A1, and Myb10-B were Tamyb10-specific markers used for determining the allelic variants of Tamyb10 (Himi et al. 2011).
DNA extraction and PCR amplification
Genomic DNA was extracted from seedlings using the method described by Gale et al. (2001). PCR reactions were performed in an Applied Biosystems 2720 thermal cycler in a total volume of 25 μl, including 2.5 μl 10× PCR buffer, 125 μM of each dNTP, 4 pmol of each primer, 1.0 U of TaKaRa LaTaq polymerase, and 50 ng of template DNA. PCR amplification were 94 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, 53 °C–68 °C for 45 s, and 72 °C for 1.5 min, with a final extension of 72 °C for 10 min. Amplified PCR fragments were separated on 1.5 % agarose gel.
DNA sequencing
The PCR products were sequenced from both strands by Huada Gene Biological Technology Co. Ltd. (http://www.genomics.cn/index.php). Sequence analysis and characterization were performed using software DNAMAN (http://www.lynon.com).
Statistical analysis
Analysis of variance was conducted by PROC MIXED in the Statistical Analysis System (SAS Institute, 8.0) with genotype clusters indicated by two types of fragments, which were amplified with STS marker Tamyb10D, as a categorical variable to derive mean GI value from each cluster and to test significant levels. The genotype clusters were treated as fixed effects, while genotypes nested in clusters and years were treated as random. Pearson’s linear correlation coefficients for GI between years were obtained by SAS PROC CORR. Significance of the association between Tamyb10-D1 and PHS tolerance in the RIL population was evaluated using general linear model (GLM) analysis, based on the phenotypic variation in GI explained by Tamyb10D estimated.
Results
Amplification and sequence analysis of three Tamyb10 homologs in varieties with different PHS tolerance
Full sequences of three Tamyb10 homologs were isolated using genome-specific primers (Table 2). Firstly, Tamyb10-A1 was amplified with the primer sets Tamyb10-AF1/R1, Tamyb10-AF2/R2, and Tamyb10-AF3/R3 in 10 bread wheats with different PHS tolerance. Sequence alignment showed no difference among the 10 bread wheat varieties, but the full sequence of these wheat varieties had 12 SNPs compared with the Tamyb10-A1 (AB191458) (2 SNPs located in exons and 10 in introns); for two SNPs in exons, one G to T change at position 177 bp in the first exon did not induce any change of amino acids, whereas the other G to A at position 2957 bp in the third exon induced the change of amino acid. The other 10 SNPs were located in the first and second introns. In addition, two 1-bp insertions (T and A) were found in the position of 1528 and 2354 bp in the introns.
The Tamyb10-B1 was amplified from the 10 bread wheats with the primer sets Tamyb10-BF1/R1, Tamyb10-BF2/R2, and Tamyb10-BF3/R3, respectively. For the PCR fragment amplified with primer sets Tamyb10-BF1/R1 and Tamyb10-BF3/R3, the sequence alignment showed that cultivar Jimai 19 had very similar sequence with Yangxiaomai except that Jimai 19 has two 6-bp insertions located in 368 and 399 bp, respectively. In addition, compared with the Tamyb10-B1 (AB191459.1), the sequence stitching amplified with primer set Tamyb10-BF1/R1 and Tamyb10-BF2/R2, in 10 bread wheat varieties, had 5 SNPs and 2-bp (GT) insertion, of which 2 were located in the first exon, i.e., G to A at position 41 bp that did not induce any change of amino acid and A to T at position 179 bp, while other three located in the introns, i.e., A to T at position 179 bp, A to G at position 685 bp, and C to T at position 1147 bp. The 2-bp insertion (GT) at position 286 bp was also located in the first intron. Polymorphic fragment was detected in the 10 bread wheats with different PHS tolerance amplified with primer set Tamyb10-BF3/R3. A 534-bp fragment was amplified in varieties Xiaobaiyuhua, Jimai 19, and Zhongyou 9507, whereas a 515-bp fragment was detected in the other varieties. Sequence alignment showed a 19-bp deletion in exon 2 of Tamyb10-Ba, comprising five GCC repeats and four bases of GACG, and caused a frame shift in its open-reading frame. This result was described in Himi et al. (2011); compared with Tamyb10-Ba, Tamyb10-Bb had a 19-bp insertion. Therefore, varieties Xiaobaiyuhua, Jimai 19, and Zhongyou 9507 amplified with a 534-bp fragment had allele Tamyb10-Bb, while the others amplified with a 515-bp fragment were Tamyb10-Ba.
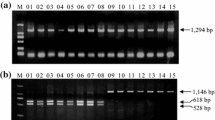
The full sequence of Tamyb10-D1 was amplified from the 10 bread wheats with the genome-specific primer sets Tamyb10-DF1/R1, Tamyb10-DF1/R2, and Tamyb10-DF3/R3. The polymorphism was detected from the PCR products of primer sets Tamyb10-DF1/R2 and Tamyb10-DF3/R3 in agarose gel but not from Tamyb10-DF1/R1. A fragment (1419 bp) of Tamyb10-D1 was amplified in the 10 bread wheats by the primer set Tamyb10-DF1/R1. Compared with the Tamyb10-Db (AB191460, red-grained wheat Chinese spring), the part sequence of Tamyb10-D1 had five SNPs; among them, G to A at position 2160 bp was located in the first exon that changed the triplet code AGA (polar uncharged amino acid glycine) into AAA (nonpolar alanine), while the other four, C to T, G to A, T to C, and T to A at positions 2637, 2698, 2823, and 3220 bp, respectively, were located in the second intron. Two kinds of fragments were detected with the primer set Tamyb10-DF1/R2. A 1629- and a 1178-bp fragment were amplified in Zhou8425B, Xiaobaiyuhua, and Yangxiaomai, whereas only a 1178-bp fragment was amplified in the other seven varieties; sequence alignment showed that the 1178-bp fragment was nonspecific amplification product, but the 1629-bp fragment was specific amplification fragment (Fig. 1). For the primer set Tamyb10-DF3/R3, the specific PCR products were obtained from varieties Zhou 8425B, Xiaobaiyuhua, and Yangxiaomai, but no PCR fragment product was detected from the other seven.
PCR fragments amplified with Tamyb10-DF1/R2 in 10 Chinese wheat varieties with different PHS tolerance. The full sequence of Tamyb10-D had been subcloned in varieties Zhou 8425B, Xiaobaiyuhua, and Yangxiaomai with the primer sets Tamyb10-DF1/R2. M Trans 2 k, 1 Zhou 8425B (average germination index 0.56), 2 Jimai 19 (0.58), 3 Jing 411(0.64), 4 Zhongyou 9507 (0.71), 5 Hengshui 7228 (0.68), 6 Yumai 18 (0.07), 7 Xiaoyuhua (0.10), 8 Xiaobaiyuhua (0.04), 9 Xiaoyan 6 (0.14), 10 Yangxiaomai (0.08). Note that the number in the bracket indicates the average GI values
The full sequence of Tamyb10-D1 was amplified in Zhou 8425B, Xiaobaiyuhua, and Yangxiaomai, which had 99.58 % similarity with that of Tamyb10-Db; therefore, varieties Zhou 8425B, Xiaobaiyuhua, and Yangxiaomai had the allele Tamyb10-Db. In the other seven varieties, no specific PCR fragments were detected with the primer sets Tamyb10-DF1/R2 and Tamyb10-DF3/R3; these varieties had the allele Tamyb10-Da, in which just a 1419-bp fragment was amplified with the primer set Tamyb10-DF1/R1.
Development and validation of Tamyb10D STS marker for PHS resistance
Based on the sequence analysis, a STS marker of Tamyb10-D1, designated as Tamyb10D (primer set Tamyb10-DF1/R2), was developed and used for association analysis with 103 Chinese varieties and advanced lines. The PCR amplification indicated two types of fragments (1629- and 1178-bp fragments and 1178-bp fragment) amplified with primer set Tamyb10-DF1/R2 (Table 2 and Fig. 1). Among the 103 varieties and lines tested, 23 were the genotype of Tamyb10-D1b amplified with 1629- and 1178-bp fragments, whereas 80 had Tamyb10-D1a with only one 1178-bp fragment (Table 1 and Fig. 2), which accounted for 22.3 and 77.7 %, and had an average GI values of 0.245 and 0.398, respectively. The GI values of the 103 varieties were consistent over the 2 years (r = 0.966, P < 0.0001), with mean value and standard deviation being 0.361 ± 0.2 in 2006 and 0.359 ± 0.19 in 2007, respectively. Analysis of variance in Tamyb10-D1 indicated significant differences between two clusters for GI (P = 0.00012). The genotypes with 1178-bp fragment were more susceptible to PHS with an average GI value of 0.398, compared with that of 1178- and 1629-bp fragments, showing more tolerance to PHS with an average GI value of 0.245. This indicated that the genotypes with the 1178- and 1629-bp fragments were more resistant to PHS than those with the 1178-bp fragment.
Association between PHS tolerance (germination rate) and the size of PCR fragments amplified with Tamyb10D in 103 white-grained bread wheat varieties. The white columns indicate the number of accessions with 1629 bp, and the black columns indicate the number of accessions with no specific amplified fragment
To further confirm the association, a RIL population developed from the cross of Yangxiaomai/Zhongyou 9507 was genotyped using Tamyb10D (Fig. 3). Statistical analysis confirmed the significant association (P < 0.001) of allelic variations of Tamyb10-Db with GI value and PHS tolerance. In this population, Tamyb10-D1 gene explained 13.7, 4.7, and 9.8 % of the phenotypic variations in Shijiazhuang, Beijing, and the averaged data from those environments, respectively, based on the test of STS marker Tamyb10D (Table 3).
Characterization of Tamyb10 allelic variants
The allelic variants of Tamyb10-A1 and Tamyb10-B1 were identified with the primer sets Myb10-A1, Myb10-A2, Myb10-A3, and Myb10-B (Himi et al. 2011). The alleles of Tamyb10-D1 were detected with the primer set Tamyb10-DF1/R2. Firstly, three primer sets Myb10-A1, Myb10-A2, and Myb10-A3 were used to identify the allelic variants of Tamyb10-A1 in the 103 varieties with different dormancy level. Thirty-eight Tamyb10-A1b varieties showed a 665-bp fragment with primer set Myb10-A1 and a 565-bp fragment with primer set Myb10-A3 but not with primer set Myb10-A2 (Table 4 and Fig. 4a). Sixty-five Tamyb10-A1a varieties showed a 665-bp fragment with primer set Myb10-A1, 636-bp fragment with primer set Myb10-A2, and 565- or 565- and 2750-bp fragments with primer set Myb10-A3 (Table 4 and Fig. 4a). Among them, five varieties including Lumai 21, Nvermai, Chuan 362, Shan 253, and PH82–2 had the heterozygote fragments with 2750 and 565 bp using the primer set Myb10-A3. Other three Tamyb10-A1a varieties Zheng 366, Yumai 47, and Waitoubai showed amplified fragments with primer sets Myb10-A2 and Myb10-A3 but not with primer set Myb10-A1 (Fig. 4a). Thirty-eight Tamyb10-A1b varieties had mean GI value and standard deviation of 0.390 ± 0.028, and 65 Tamyb10-A1a varieties had mean GI value and standard deviation of 0.342 ± 0.024, and analysis of variance indicated that there were not significant differences between two clusters for GI.
The primer set Myb10-B was used to identify the allelic variants of Tamyb10-B1 in these germplasm. Two types of fragments (282 and 263 bp) were detected in 103 varieties (Fig. 4b). Genotypes with a 282-bp fragment were the allele Tamyb10-B1b, whereas genotypes with 263 bp were Tamyb10-B1a. Forty-eight Tamyb10-B1b varieties had mean GI value and standard deviation of 0.491 ± 0.020 and 55 Tamyb10-B1a varieties had mean GI value and standard deviation of 0.245 ± 0.020 (Table 4), and analysis of variance indicated significant differences between two clusters for GI (P = 0.9 × 10−13). The data showed that the allelic variants of Tamyb10-B1 were associated with PHS tolerance.
With the marker Tamyb10D, a 1629-bp fragment was amplified in genotypes of Tamyb10-D1b, while nonspecific amplification was found in the genotype Tamyb10-Da. Twenty-three Tamyb10-D1b varieties and 80 Tamyb10-D1a varieties were detected (Fig. 1 and Table 4).
In the 103 Chinese bread wheats with different PHS tolerance, 8 Tamyb10 genotypes (Tamyb10-A1, Tamyb10-B1, and Tamyb10-D1 loci) were detected, namely, aaa, aab, aba, abb, baa, bab, bba, and bbb, which showed a continuous color change with increase of dominant allele “b” (Fig. 5). Multiple comparisons indicated that the average GI was significantly different among genotypes with different allele combinations such as aaa and aba, aaa and bba, aab and aba, aab and bba, aba and abb, aba and bab, abb and bba, baa and bba, and bab and bba (P < 0.01). As shown in Table 5, the genotypes with the allele combinations aaa, aab, abb, baa, and bab showed resistance to PHS with average GI values of 0.264, 0.198, 0.275, 0.283, and 0.172, respectively, whereas the genotypes with aba and bba showed susceptible to PHS with average GI values of 0.508 and 0.519, respectively (Table 5).
Discussion
An association between grain color and depth of dormancy has been noted in a Vp-1 mutant of maize and in transparent testa (tt) mutants of Arabidopsis (McCarty et al. 1991; Debeaujon et al. 2000). Normally, red-grained varieties are usually more tolerant to PHS than white-grained wheat varieties (Flintham 2000; Warner et al. 2000; Himi et al. 2002); however, it is proven that the statement is not all true in the present study. Some white-grained varieties, such as Xumai 954, Fengchan 3, Xinmai 13, Yumai 18, and Xiaoyuhua, had higher PHS tolerance with germination rates below 10 % (Table 1), although they had an allele combination of aaa. White-grained wheat varieties are homozygous for the recessive alleles of R-1 gene, while red-grained wheat varieties are heterozygous or homozygous for the dormant alleles of R-1 gene ((McIntosh et al. 1998), and no amplified fragments from genomic DNA and cDNA of white-grained wheats were obtained by PCR using specific primers of Tamyb10-Da before (Himi et al. 2011). In the present study, a part of genomic sequences of Tamyb10-Da (1419 bp) were amplified fortunately from bread wheat (R-D1a varieties) with higher GI values. Compared with the Tamyb10-D1b (AB191460), ORF of Tamyb10-Da also had very high similarity with Tamyb10-B1a besides sequence of AB191460. Among the 103 Chinese bread wheat varieties, only 23 (average GI value was 0.245) were the allele Tamyb10-D1b, and the others (average GI value was 0.398) were Tamyb10-D1a with only one 1419-bp fragment amplified, suggesting that mutation locus of Tamyb10-D1a might occur in the region between 1419 and 1629 bp, leading an early termination in the coding region; that is why the nonspecific PCR product were amplified with the primer sets Tamyb10-DF1/R2 and Tamyb10-DF3/R3 in these varieties.
Chinese spring with deletions of chromosome 3DL produces seeds with white color (Himi and Noda 2004), indicating that deletion of either the DFR gene or Myb/c or TaVp-1D or all of these three genes on chromosome 3D have loss or gain of functions in grain color (Xia et al. 2008); moreover, in red-grained wheat, Tamyb10-D1 has the greatest effect on PHS resistance, followed by Tamyb10-B1, and Tamyb10-A1 had the least effect (Wang et al. 2014). In this germplasm, there is no significant difference of GI between Tamyb10-A1b and Tamyb10-A1a genotypes, but for Tamyb10-B1 and Tamyb10-D1, the significant difference of GI is present between Tamyb10-B1a and Tamyb10-B1b (F = 1.400, P = 0.9 × 10−13) and between Tamyb10-D1b and Tamyb10-D1a (F = 0.296, P = 0.00012), respectively. Moreover, varieties with allele Tamyb10-B1a were more resistant than that with Tamyb10-B1b, and the varieties with Tamyb10-D1b were more resistant than those with Tamyb10-D1a. The above data showed that Tamyb10-B1 had the greatest effect on seed dormancy, followed by Tamyb10-D1, and Tamyb10-A1 had the least effect, and these also showed that there is no correlation between the numbers of R-b (including R-A1b, R-B1b, and R-D1b) and the level of seed dormancy in a variety. It may be a reason that the red-grained wheat varieties are usually more tolerant to PHS than white-grained wheat varieties, but not all the red grained wheats had higher PHS resistance than white grained. In this study, there was another interesting result; the RIL population of Yangxiaomai/Zhongyou 9507 was also used to confirm the association of Tamyb10-B variations (Tamyb10-Ba and Tamyb10-Bb) and GI values, but statistical analysis showed no significant differences of GI (F = 6.67, P = 0.254) between two genotypes.
In our previous study, STS markers Vp1A3 and Vp1B3 were associated with seed dormancy in Chinese wheats with different GI values (Yang et al. 2007, 2014); the efficiency of marker-assisted selection for PHS-resistant varieties was improved using Vp1A3 and Vp1B3. The allele combinations TaVp-1Agm and TaVp-1Bb, TaVp-1Agm and TaVp-1Ba, TaVp-1Aim and TaVp-1Bb, and TaVp-1Aam and TaVp-1Bb could confer higher PHS resistance (Yang et al. 2014). But, some varieties such as Suiningtuotuomai (average GI = 0.10) and Waitoubai (average GI = 0.07) with the alleles TaVp-1Aam and TaVp-1Bc did not carry any of the PHS-resistant allele combinations but also had the higher PHS resistance (Yang et al. 2014). Interestingly, Suiningtuotuomai and Waitoubai both carry Tamyb10-Db amplified with the STS marker Tamyb10D associated with PHS tolerance. Therefore, for PHS resistance breeding in wheat, using STS marker combinations (Vp1A3, Vp1B3, and Tamyb10D) will improve the efficiency of selection very much. Because Vp1A3 and Vp1B3 are also associated with embryo-imposed dormancy, and Tamyb10D is associated with coat-imposed dormancy, the mechanism of seed dormancy can be divided into embryo-imposed and coat-imposed dormancy.
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Bailey PC, McKibbin RS, Lenton JR, Holdsworth MJ, Flintham JE, Gale MD (1999) Genetic map locations for orthologous Vp1 genes in wheat and rice. Theor Appl Genet 98:281–284
Bentsink L, Jowett J, Hanhart CJ, Koornneef M (2006) Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci U S A 103:17042–17047
Carson CB, Hattori T, Rosenkrans L, Vasil V, Peterson PA, McCarty DR (1997) The quiescent/colorless alleles of viviparous1 show that the conserved B3 domain of Vp1 is not essential for ABA-regulated gene expression in the seed. Plant J 12:1231–1240
Chang C, Feng JM, Si HQ, Yin B, Zhang HP, Ma CX (2010) Validating a novel allele of viviparous-1 (Vp-1Bf) associated with high seed dormancy of Chinese wheat landrace, Wanxianbaimaizi. Mol Breeding 25:517–525
Chang C, Zhang HP, Zhao QX, Feng JM, Si HQ, Lu J, Ma CX (2011) Rich allelic variations of Viviparous-1A and their associations with seed dormancy/pre-harvest sprouting of common wheat. Euphytica 179:343–353
Chopra S, Athma P, Peterson T (1996) Alleles of the maize P gene with distinct tissue specificities encode Myb-homologous proteins with C-terminal replacements. Plant Cell 8:1149–1158
Debeaujon I, Leon-Kloosterziel KM, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122:403–414
Flintham J (2000) Different genetic components control coat-imposed and embryo-imposed dormancy in wheat. Seed Sci Res 10:43–50
Gale KR, Ma W, Zhang W, Rampling L, Hill AS, Appels R, Morris P, Morrel M (2001) Simple high-throughput DNA markers for genotyping in wheat. In: Eastwood R et al. (eds) 10th Australian Wheat Breeding Assembly Proceedings, pp 26–31
Giraudat J, Hauge BM, Valon C, Smalle J, Parcy F, Goodman HM (1992) Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4:1251–1261
Groos C, Gay G, Perretant MR, Gervais L, Bernard M, Dedryver F, Charmet G (2002) Study of the relationship between pre-harvest sprouting and grain color by quantitative trait loci analysis in a white × red grain bread-wheat cross. Theor Appl Genet 104:39–47
Gubler F, Millar AA, Jacobsen JV (2005) Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8:183–187
Hattori T, Terada T, Hamasuna ST (1994) Sequence and functional analyses of the rice gene homologous to the maize Vp1. Plant Mol Biol 24:805–810
Hattori T, Vasil V, Rosenkrans L, Hannah LC, McCarty DR, Vasil IK (1992) The Viviparous-1 gene and abscisic acid activate the C1 regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Gene Dev 6:609–618
Himi E, Maekawa M, Miura H, Noda K (2011) Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theor Appl Genet 122:1561–1576
Himi E, Mares DJ, Yanagisawa A, Noda K (2002) Effect of grain color gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J Exp Bot 53:1569–1574
Himi E, Noda K (2004) Isolation and location of three homoeologous dihydroflavonol-4-reductase (DFR) genes of wheat and their tissue-dependent expression. J Exp Bot 55:365–375
Himi E, Noda K (2005) Red grain color gene (R) of wheat is a Myb-type transcription factor. Euphytica 143:239–242
Himi E, Yamashita Y, Haruyama N, Yanagisawa T, Maekawa M, Taketa S (2012) Ant28 gene for proanthocyanid in synthesis encoding the R2R3 MYB domain protein (Hvmyb10) highly affects grain dormancy in barley. Euphytica 188:141–151
Holton TA, Cornish EC (1995) Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7:1071–1083
Koornneef M, Hanhart CJ, Hilhorst HWM, Karssen CM (1989) In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol 90:463–469
Li CD, Ni PX, Francki M, Hunter A, Zhang Y, Schibeci D, Li H, Tarr A, Wang J, Cakir M, Yu J, Bellgard M, Lance R, Appels R (2004) Genes controlling seed dormancy and pre-harvest sprouting in a rice-wheat-barley comparison. Funct Integr Genomics 4:84–93
Liu YX, Koornneef M, Soppe WJJ (2007) The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell 19:433–444
McCarty DR, Carson CB, Stinard PS, Robertson DS (1989) Molecular analysis of Viviparous-1: an abscisic acid insensitive mutant of maize. Plant Cell 1:523–532
McCarty DR, Hattori T, Carson CB, Vasil V, Lazar M, Vasil IK (1991) The Viviparous-1 developmental gene of maize encodes a novel transcriptional activator. Cell 66:895–905
McIntosh RA, Hart GE, Devos KM, Gale MD, Rogers WJ (1998) Catalogue of gene symbols for wheat. In: Proceedings of the ninth international wheat genetics symposium, Canada, University of Saskatchewan Extension Press
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers J, Morris CF, Somers DJ, Appels R, Devos KM (2010) Catalogue of Gene Symbols for Wheat National BioResource Project. Committee for the National BioResource Project (NBRP)/KOMUGI, Tokyo. www.shigen.nig.ac.jp/wheat/komugi/genes/symbolClassList.jsp
McKibbin RS, Wilkinson MD, Bailey PC, Flintham JE, Andrew LM, Lazzeri PA, Gale MD, Lenton JR, Holdsworth MJ (2002) Transcripts of Vp-1 homeologues are misspliced in modern wheat and ancestral species. Proc Natl Acad Sci U S A 99:10203–10208
Mol J, Grotewold E, Koes R (1998) How genes paint flowers and seeds. Trends Plant Sci 3:212–217
Nakamura S, Toyama T (2001) Isolation of a VP1 homologue from wheat and analysis of its expression in embryos of dormant and non-dormant cultivars. J Exp Bot 52:875–876
Robertson DS (1955) The genetics of vivipary in maize. Genetics 40:745–760
Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci U S A 98:11806–11811
Sun YW, Jones HD, Yang Y, Dreisigacker S, Li SM, Chen XM, Shewry PR, Xia LQ (2012) Haplotype analysis of Viviparous-1 gene in CIMMYT elite bread wheat germplasm. Euphytica 186:25–43
Tan MK, Sharp PJ, Lu MQ, Howes N (2006) Genetics of grain dormancy in a white wheat. Aust J Agric Res 57:1157–1165
Wang GP, Bi HH, Sun YW, Wang CS, Xia LQ (2014) Characterization of Tamyb10 haplotypes and their association with pre-harvest sprouting resistance in a set of Chinese red-grained wheats. Acta Agron Sin 40:984–993
Warner R, Kudrna D, Spaeth S, Jones S (2000) Dormancy in wheat grain-mutant of Chinese spring wheat (Triticum aestivum L.). Seed Sci Res 10:51–60
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126(2):485–493
Xia LQ, Ganal MW, Shewry PR, He ZH, Yang Y, Röder M (2008) Evaluation of the Viviparous-1 gene alleles in the European wheat varieties. Euphytica 159:411–417
Xia LQ, Yang Y, Ma YZ, Chen XM, He ZH, Roder MS, Jones HD, Shewry PR (2009) What can the Viviparous-1 gene tell us about wheat pre-harvest sprouting? Euphytica 168:385–394
Yang Y, Chen XM, He ZH, Xia LQ, Röder M (2009) Distribution of Vp-1 alleles in Chinese white-grained landraces, historical, and current wheat cultivars. Cereal Res Comm 37:169–177
Yang Y, Zhao XL, Xia LQ, Chen XM, Xia XC, Yu Z, He ZH (2007) Development and validation of a Viviparous-1 STS marker for pre-harvest sprouting tolerance in Chinese wheats. Theor Appl Genet 115:971–980
Yang Y, Zhang CL, Liu SX, Sun YQ, Meng JY, Xia LQ (2014) Characterization of the rich haplotypes of Viviparous-1A in Chinese wheats and development of a novel sequence-tagged site marker for pre-harvest sprouting resistance. Mol Breeding 33:75–88
Zhang YJ, Miao XL, Xia XC, He ZH (2014) Cloning of seed dormancy gene (TaSdr) associated with tolerance to pre-harvest sprouting in common wheat and development of a functional marker. Theor Appl Genet 127:855–866
Zheng J, Chen F, Wang Z, Cao H, Li X, Deng X, Soppe WJJ, Li Y, Liu Y (2012) A novel role for histone methyltransferase KYP/SUVH4 in the control of Arabidopsis primary seed dormancy. New Phytol 193:605–616
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (31160247, 31260320), Innovative Research Group Fund of Inner Mongolia Agriculture University (NDPYTD2010-3), and Excellent Young Scientist Foundation of Inner Mongolia Agricultural University of China (2014XYQ-18).
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Wang and X. L. Wang contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, Y., Wang, X.L., Meng, J.Y. et al. Characterization of Tamyb10 allelic variants and development of STS marker for pre-harvest sprouting resistance in Chinese bread wheat. Mol Breeding 36, 148 (2016). https://doi.org/10.1007/s11032-016-0573-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-016-0573-9