Abstract
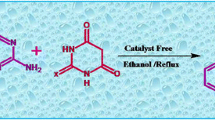
In this study, the one-pot reaction between primary amines, 1,1-bis-(methylthio)-2-nitroethene, ninhydrin, and barbituric acid as an enolizable C–H-activated compound provides a simple method for the preparation of 5-(2-(alkylamino)-1,3-dioxo-2,3-dihydro-1H-inden-2-yl)-6-hydroxypyrimidine-2,4(1H,3H)-dione derivatives with potential synthetic and pharmacological interest. This reaction shows attractive characteristics, such as substrate availability, good yields, existence of numerous hydrogen-bonding possibilities in product, and its mild conditions in ethanol media.
Graphic abstract








Similar content being viewed by others

References
Li B, Webster TJ (2018) Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopedic infections. J Orthop Res 36:22–32
Ajani OO, Isaac JT, Owoeye TF, Akinsiku AA (2015) Exploration of the chemistry and biological properties of pyrimidine as a privilege pharmacophore in therapeutics. Int J Biol Chem 9:148–177
Lagoja IM (2005) Pyrimidine as constituent of natural biologically active compounds. Chem Biodivers 2:1–50
Sharma P, Rane N, Gurram VK (2004) Synthesis and QSAR studies of pyrimido [4,5-d] pyrimidine-2, 5-dione derivatives as potential antimicrobial agents. Bioorg Med Chem Lett 14:4185–4190
Basavaraja HS, Sreenivasa GM, Jayachandran E (2005) Synthesis and biological activity of novel pyrimidino imidazolines. Indian J Heterocycl Chem 15:69
Kaldrikyan MA, Grigoryan LA, Geboyan VA, Arsenyan FG, Stepanyan GM, Garibdzhanyan BT (2000) Synthesis and antitumor activity of some disubstituted 5-(3-methyl-4-alkoxybenzyl) pyrimidines. Pharm Chem J 34:521–524
Nezu Y, Miyazaki M, Sugiyama K, Kajiwara I (1996) Dimethoxypyrimidines as novel herbicides. Part 1. Synthesis and herbicidal activity of dimethoxyphenoxyphenoxypyrimidines and analogues. Pestic Sci 47:103–113
Wannachaiyasit S, Chanvorachote P, Nimmannit U (2008) A novel anti-HIV dextrin–zidovudine conjugate improving the pharmacokinetics of zidovudine in rats. AAPS PharmSciTech 9:840
Hannah DR, Stevens MF (2003) Structural studies on bioactive compounds. Part 38.1 reactions of 5-aminoimidazole-4-carboxamide: synthesis of imidazo[1,5-a]quinazoline-3-carboxamides. J Chem Res 2003:398–401
Balzarini J, McGuigan C (2002) Bicyclic pyrimidine nucleoside analogues (BCNAs) as highly selective and potent inhibitors of varicella-zoster virus replication. J Antimicrob Chemother 50:5–9
Lee HW, Kim BY, Ahn JB, Kang SK, Lee JH, Shin JS, Yoon SS (2005) Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur J Med Chem 40:862–874
Gupta AK, Kayath HP, Ajit S, Geeta S, Mishra KC (1994) Anticonvulsant activity of pyrimidine thiols. Indian J Pharmacol 26:227
Abu-Hashem A, El-Shehry M, Badria F (2010) Design and synthesis of novel thiophenecarbohydrazide, thienopyrazole and thienopyrimidine derivatives as antioxidant and antitumor agents. Acta Pharm 60:311–323
Rahaman SA, Pasad YR, Kumar P, Kumar B (2009) Synthesis and anti-histaminic activity of some novel pyrimidines. Saudi Pharm J 17:255–258
Rodrigues ALS, Rosa JM, Gadotti VM, Goulart EC, Santos MM, Silva AV, Santos ARS (2005) Antidepressant-like and antinociceptive-like actions of 4-(4′-chlorophenyl)-6-(4″-methylphenyl)-2-hydrazinepyrimidine Mannich base in mice. Pharmacol Biochem Behav 82:156–162
Choi Y, Kim H, Park SB (2019) A divergent synthetic pathway for pyrimidine-embedded medium-sized azacycles through an N-quaternizing strategy. Chem Sci 10:569–575
Jain KS, Chitre TS, Miniyar PB, Kathiravan MK, Bendre VS, Veer VS, Shishoo CJ (2006) Biological and medicinal significance of pyrimidines. Curr Sci 90:793
Brogden RN, Carmine AA, Heel RC, Speight TM, Avery GS (1982) Trimethoprim: a review of its antibacterial activity, pharmacokinetics and therapeutic use in urinary tract infections. Drugs 23:405–430
Millan MJ, Cussac D, Milligan G, Carr C, Audinot V, Gobert A, Nicolas JP (2001) Antiparkinsonian agent piribedil displays antagonist properties at native, rat, and cloned, human α2-adrenoceptors: cellular and functional characterization. J Pharmacol Exp Ther 297:876–887
Moffett BS, Weingarten MM, Galati M, Placencia JL, Rodman EA, Riviello JJ, Kayyal SY (2018) Phenobarbital population pharmacokinetics across the pediatric age spectrum. Epilepsia 59:1327–1333
Ahmed N (2016) Synthetic advances in the indane natural product scaffolds as drug candidates: a review. Stud Nat Prod Chem 51:383–434
Catto M, Aliano R, Carotti A, Cellamare S, Palluotto F, Purgatorio R, Campagna F (2010) Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1, 3-diones and indol-2-aryldiazenylmethylene-3-ones as β-amyloid aggregation inhibitors. Eur J Med Chem 45:1359–1366
Prabhakar KR, Veerapur VP, Bansal P, Vipan KP, Reddy KM, Barik A, Unnikrishnan MK (2006) Identification and evaluation of antioxidant, analgesic/anti-inflammatory activity of the most active ninhydrin–phenol adducts synthesized. Bioorg Med Chem 14:7113–7120
Ziarani GM, Lashgari N, Azimian F, Kruger HG, Gholamzadeh P (2015) Ninhydrin in synthesis of heterocyclic compounds. ARKIVOK 6:1–139
ElKalyoubi S, Fayed E (2016) Synthesis and evaluation of antitumour activities of novel fused tri-and tetracyclic uracil derivatives. J Chem Res 40:771–777
Naguib BH, El-Nassan HB, Abdelghany TM (2017) Synthesis of new pyridothienopyrimidinone derivatives as Pim-1 inhibitors. J Enzyme Inhib Med Chem 32:457–467
Squarcialupi L, Betti M, Catarzi D, Varano F, Falsini M, Ravani A, Varani K (2017) The role of 5-arylalkylamino-and 5-piperazino-moieties on the 7-aminopyrazolo[4,3-d]pyrimidine core in affecting adenosine A1 and A2A receptor affinity and selectivity profiles. J Enzyme Inhib Med Chem 32:248–263
Elkanzi NAA (2013) Synthesis of pyrimidine and pyrimidinthione. Heterocycl Lett 3:247–268
Kidder GW, Dewey VC (1949) The biological activity of substituted pyrimidines. J Biol Chem 178:383–387
Zhang L, Dong J, Xu X, Liu Q (2016) Chemistry of ketene N, S-acetals: an overview. Chem Rev 116:287–322
Mohammadi A, Bayat M, Nasri S (2019) Catalyst-free four-component domino synthetic approach toward versatile multicyclic spirooxindole pyran scaffolds. RSC Adv 9:16525–16533
Alizadeh A, Zarei A, Rezvanian A (2011) A novel and one-pot multicomponent approach to the synthesis of dihyroindeno[1,2-b]pyrroles and indeno[2′,1′:4,5]pyrrolo[1,2-a]-fused 1, 3-diazaheterocycles. Synthesis 2011:497–501
Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kochia, K., Bayat, M., Nasri, S. et al. Synthesis of new pyrimidine-containing compounds: 5-(2-(alkylamino)-1,3-dioxo-2,3-dihydro-1H-inden-2-yl)-6-hydroxypyrimidine-2,4(1H,3H)-dione derivatives. Mol Divers 24, 1015–1024 (2020). https://doi.org/10.1007/s11030-019-10009-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-10009-w