Abstract
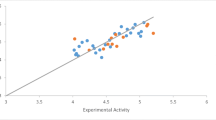
In current study, antitumor activity of two series of the newly synthesized spiropyrroloquinoline isoindolinone and spiropyrroloquinoline aza-isoindolinone scaffolds was evaluated against three human breast normal and cancer cell lines (MCF-10A, MCF-7 and SK-BR-3) and compared with cytotoxicity values of doxorubicin and colchicine as the standard drugs. It was found that several compounds were endowed with cytotoxicity in the low micromolar range. Among these two series, compounds 6i, 6j, 6k and 7l, 7m, 7n, 7o containing 3-ethyl-1H-indole moiety were found to be highly effective against both cancer cell lines ranging from \(0.080 \pm 0.001\) to \(11.91 \pm 1.39\,\upmu \hbox {M}\) in comparison with the corresponding analogs. Compared with human cancer cells, the most potent compounds did not show high cytotoxicity against human breast normal MCF-10A cells. Generally, most of the evaluated compounds 6a–l and 7a–o series showed more antitumor activity against SK-BR-3 than MCF-7 cells. Moreover, comparative molecular field analysis (CoMFA) as a popular tools of three-dimensional quantitative structure–activity relationship (3D-QSAR) studies was carried out on 27 spiropyrroloquinolineisoindolinone and spiropyrroloquinolineaza-isoindolinone derivatives with antitumor activity against on SK-BR-3 cells. The obtained CoMFA models showed statistically excellent performance, which also possessed good predictive ability for an external test set. The results confirm the important effect of molecular steric and electrostatic interactions of these compounds on in vitro cytotoxicity against SK-BR-3.







Similar content being viewed by others
References
Edmondson S, Danishefsky SJ, Sepp-Lorenzino L, Rosen N (1999) Total synthesis of spirotryprostatin A, leading to the discovery of some biologically promising analogues. J Am Chem Soc 121:2147–2155. doi:10.1021/ja983788i
Zhang Z, Chu ZI, Liu JJ, Ding Q, Zhang J, Bartkovitz D, Jiang N, Karnachi P, So SS, Tovar C, Filipovic Z, Higgins B, Glenn K, Packman K, Vassilev L, Graves B (2014) Discovery of potent and orally active p53- MDM2 inhibitors RO5353 and RO2468 for potential clinical development. ACS Med Chem Lett 5:124–127. doi:10.1021/ml400359z
Lunn MR, Root DE, Martino AM, Flaherty SP, Kelley BP, Coovert DD, Burghes AH, Thi Man N, Morris GE, Zhou J, Androphy EJ, Sumner CJ, Stockwell BR (2004) Indoprofen upregulates the survival motor neuron protein through a cyclooxygenase-independent mechanism. Chem Biol 11:1489–1493. doi:10.1016/j.chembiol.2004.08.024
Comins DL, Schilling S, Zhang Y (2005) Asymmetric synthesis of 3-substituted isoindolinones: application to the total synthesis of (+)-lennoxamine. Org Lett 7:95–98. doi:10.1021/ol047824w
Bayod Jasanaba M-S, Ribas Bueno C (2009) Process for synthesis and purification of anhydrous crystalline S- zopiclone. PCT Patent EP 2058313:A2
Goto G, Saji Y (1988) Isoindolinone derivatives, production and use thereof. US Patent 4778801 A
Wrobel J, Dietrich A, Woolson SA, Millen J, McCaleb M, Harrison MC, Hohman TC, Sredy J, Sullivan D (1992) Novel spirosuccinimides with incorporated isoindolone and benzisothiazole 1,1-dioxide moieties as aldose reductase inhibitors and antihyperglycemic agents. J Med Chem 35:4613–4627. doi:10.1021/jm00102a016
Breytenbach JC, van Dyk S, van Den Heever I, Allin SM, Hodkinson CC, Northfield CJ, Page MI (2000) Synthesis and antimicrobial activity of some isoindolin-1-ones derivatives. Bioorg Med Chem Lett 10:1629–1631. doi:10.1016/S0960-894X(00)00306-1
Maugeri C, Alisi MA, Apicella C, Cellai L, Dragone P, Fioravanzo E, Florio S, Furlotti G, Mangano G, Ombrato R, Luisi R, Pompei R, Rincicotti V, Russo V, Vitiello M, Cazzolla N (2008) New anti-viral drugs for the treatment of the common cold. Bioorg Med Chem 16:3091–3107. doi:10.1016/j.bmc.2007.12.030
Yu W, Guo Z, Orth P, Madison V, Chen L, Dai C, Feltz RJ, Girijavallabhan VM, Kim SH, Kozlowski JA, Lavey BJ, Li D, Lundell D, Niu X, Piwinski JJ, Popovici-Muller J, Rizvi R, Rosner KE, Shankar BB, Shih N-Y, Arshad Siddiqui M, Sun J, Tong L, Umland S, Wong MKC, Yang D-Y, Zhou G (2010) Discovery and SAR of hydantoin TACE inhibitors. Bioorg Med Chem Lett 20:1877–1880. doi:10.1016/j.bmcl.2010.01.148
Zhao XZ, Maddali K, Marchand C, Pommier Y, Burke TR Jr (2009) Diketoacid-genre HIV-1 integrase inhibitors containing enantiomeric arylamide functionality. Bioorg Med Chem 17:5318–5324. doi:10.1016/j.bmc.2009.05.008
Liu J, Wang L, Guo N, Teng Y-O, Yu P (2014) Design, synthesis and biological evaluation of the novel isoindolinone derivatives. J Chem Pharm Res 6:256–263
Hardcastle IR, Liu J, Valeur E, Watson A, Ahmed SU, Blackburn TJ, Bennaceur K, Clegg W, Drummond C, Endicott JA, Golding BT, Griffin RJ, Gruber J, Haggerty K, Harrington RW, Hutton C, Kemp S, Lu X, McDonnell JM, Newell DR, Noble ME, Payne SL, Revill CH, Riedinger C, Xu Q, Lunec J (2011) Isoindolinone inhibitors of the murine double minute 2 (MDM2)-p53 protein-protein interaction: structure-activity studies leading to improved potency. J Med Chem 54:1233–1243. doi:10.1021/jm1011929
Hardcastle IR, Ahmed SU, Atkins H, Farnie G, Golding BT, Griffin RJ, Guyenne S, Hutton C, Källblad P, Kemp SJ, Kitching MS, Newell DR, Norbedo S, Northen JS, Reid RJ, Saravanan K, Willems HM, Lunec J (2006) Small-molecule inhibitors of the MDM2-p53 protein–protein interaction based on an isoindolinone scaffold. J Med Chem 49:6209–6221. doi:10.1021/jm0601194
Canner JA, Sobo M, Ball S, Hutzen B, DeAngelis S, Willis W, Studebaker AW, Ding K, Wang S, Yang D, Lin J (2009) MI-63: a novel small-molecule inhibitor targets MDM2 and induces apoptosis in embryonal and alveolar rhabdomyosarcoma cells with wild-type p53. Br J Cancer 101:774–781. doi:10.1038/sj.bjc.6605199
Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S (2006) Structure-based design of spiro-oxindoles as potent, specific small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 49:3432–3435. doi:10.1021/jm051122a
Muller GW, Chen R, Huang SY, Corral LG, Wong LM, Patterson RT, Chen YX, Kaplan G, Stirling DI (1999) Amino-substituted thalidomide analogs: potent inhibitors of TNF-\(\alpha \) production. Bioorg Med Chem Lett 9:1625–1630. doi:10.1016/S0960-894X(99)00250-4
Lee BD, Li Z, French KJ, Zhuang Y, Xia Z, Smith CD (2004) Synthesis and evaluation of dihydropyrroloquinolines that selectively antagonize P-glycoprotein. J Med Chem 47:1413–1422. doi:10.1021/jm0303204
Lee BD, French KJ, Zhuang Y, Smith CD (2003) Development of a syngeneic in vivo tumor model and its use in evaluating a novel P-glycoprotein modulator, PGP-4008. Oncol Res 14:49–60. doi:10.3727/000000003108748603
Allingham JS, Smith R, Rayment I (2005) The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol 12:378–379. doi:10.1038/nsmb908
Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299:1743–1747. doi:10.1126/science.1081412
Chen K, Tang X-Y, Shi M (2016) Rh(II)-catalyzed formation of pyrrolo[2,3-b]quinolines from azide- methylenecyclopropanes and isonitriles. Chem Commun 52:1967–1970. doi:10.1039/C5CC09236A
Deshmukh GB, Patil NS, Gaikwad VB, Bhole AD, Patil SV (2014) Synthesis of substituted \(N\)-aryl pyrollo- quinolines and study of their antimicrobial activities. J Chem Pharm Res 6:393–399
Cramer RD, Patterson DE, Bunce JD (1988) Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 110:5959–5967. doi:10.1021/ja00226a005
Kubinyi H (2002) Comparative molecular field analysis (CoMFA). In: Encyclopedia of computational chemistry. Wiley
Zhang QY, Wan J, Xu X, Yang GF, Ren YL, Liu JJ, Wang H, Guo Y (2007) Structure-based rational quest for potential novel inhibitors of human HMG-CoA reductase by combining CoMFA 3D QSAR modeling and virtual screening. J Comb Chem 9:131–138. doi:10.1021/cc060101e
Cramer RD, Cruz P, Stahl G, Curtiss WC, Camphell B, Masek BB, Soltanshahi F (2008) Virtual screening for R-groups, including predicted \(\text{ pIC }_{50}\) contributions, within large structural databases, using topomer CoMFA. J Chem Inf Model 48:2180–2195. doi:10.1021/ci8001556
Matysiak J, Niewiadomy A (2017) QSAR models of antiproliferative activity of imidazo[2,1- b][1,3,4]thiadiazoles in various cancer cell lines. Mol Divers 21:211–218. doi:10.1007/s11030-016-9705-8
Da Cunha J, Lavaggi ML, Abasolo MI, Cerecetto H, Gonzalez M (2011) 2D- and 3D-quantitative structure- activity relationship studies for a series of phenazine \(N\),\(N\)’-dioxide as antitumour agents. Chem Biol Drug Des 78:960–968. doi:10.1111/j.1747-0285.2011.01237.x
Ou L, Han S, Ding W, Jia P, Yang B, Medina-Franco JL, Giulianotti MA, Chen J-Z, Yu Y (2011) Parallel synthesis of novel antitumor agents: 1,2,3-triazoles bearing biologically active sulfonamide moiety and their 3D- QSAR. Mol Divers 15:927–946. doi:10.1007/s11030-011-9324-3
Ghandi M, Zarezadeh N, Abbasi A (2015) One-pot synthesis of spiropyrroloquinoline-isoindolinone and their aza-analogs via Ugi-4CR/ metal-free intramolecular bisannulation process. Org Biomol Chem 13:8211–8221. doi:10.1039/C5OB01095K
Ojha PK, Mitra I, Narayan Das R, Roy K (2011) Further exploring \(r_{m}^{2}\) metrics for validation of QSPR models. Chemometr Intell Lab Syst 107:194–205. doi:10.1016/j.chemolab.2011.03.011
Golbraikh A, Tropsha A (2002) Beware of q2!. J Mol Graph Model 20:269–276. doi:10.1016/S1093-3263(01)00123-1
Wold S, Sjostrom M, Eriksson L (2001) PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab 58:109–130. doi:10.1016/S0169-7439(01)00155-1
Acknowledgements
Financial support by Radiation Application Research School of the Nuclear Science & Technology Research Institute is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadeghzadeh, M., Salahinejad, M., Zarezadeh, N. et al. Antitumor evaluation and 3D-QSAR studies of a new series of the spiropyrroloquinoline isoindolinone/aza-isoindolinone derivatives by comparative molecular field analysis (CoMFA). Mol Divers 21, 821–830 (2017). https://doi.org/10.1007/s11030-017-9778-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-017-9778-z