Abstract
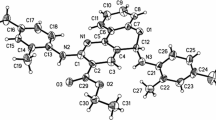
An efficient synthesis of 2-hydroxy-3-[2-oxo-2-phenylethylidene]-2-phenyl-2, 3-dihydro-4 H-furo[3, 2-c]chromene-4(2H)-one is described. This involves the reaction between dibenzoylacetylene and 4-hydroxycoumarine in the presence of NaH (10 mol %) in nearly quantitative yield. Treatment of this heterocyclic system with trimethyl chlorosilane in CHCl3 leads quantitatively to 4-oxo-3-[2-oxo-2-phenylethylidene]-2-phenyl-3H, 4H-furo[3,2-c]chromene-1-ium chloride. Direct addition of nucleophiles, such as alcohols, amines or trialkyl phosphites to this salt in water as the solvent produces functionalized 2-phenyl-4H-furo[3,2-c] chromen derivatives in excellent yields.
Similar content being viewed by others
References
Miao H, Yang Z (2000) Regiospecific carbonylative annulation of iodophenol acetates and acetylenes to construct the flavones by a new catalyst of palladium thiourea dppp complex. Org Lett 2: 1765. doi:10.1021/ol000087t
Kumar P, Bodas MS (2000) A novel synthesis of 4H-chromen-4-ones via intramolecular Wittig reaction. Org Lett 2: 3821. doi:10.1021/ol006518p
van Otterlo WAL, Lindani NE, Kuzvidza S, Morgans GL, Moleele SS, de Koning CB (2005) Ring-closing metathesis for the synthesis of 2H and 4H-chromenes. Tetrahedron 61: 9996–10006. doi:10.1016/j.tet.2005.08.020
Heravi MM, Bakhtiari K, Zadsirjan V, Bamoharram FF, Heravi OM (2007) Aqua mediated synthesis of substituted 2-amino-4H-chromenes catalyzed by green and reusable Preyssler heteropolyacid. Bioorg Med Chem Lett 17: 4262–4265. doi:10.1016/j.bmcl.2007.05.023
Chauder BA, Lopes CC, Lopes RSC, da Silva AJM, Snieckus V (1998) Phenylboronic acid-mediated synthesis of 2H-chromenes. Synthesis-Stuttgart 1998: 279. doi:10.1055/s-1998-2042
Parker AK, Mindt TL (2001) Electrocyclic ring closure of the enols of vinyl quinones. A 2H-chromene synthesis. Org Lett 3: 3875. doi:10.1021/ol0167199
Yu N, Aramini JM, Germann MW, Huang Z (2000) Reactions of salicylaldehydes with alkyl cyanoacetates on the surface of solid catalysts: syntheses of 4H-chromene derivatives. Tetrahedron Lett 41: 6993–6996. doi:10.1016/S0040-4039(00)01195-3
Hepworth JD, Gabbutt CD, Heron BM (1996) Pyrans and their benzo derivatives: synthesis. In: Katritzky AR, Rees CW, Scriven EFV (eds) Comprehensive heterocyclic chemistry II, vol 5. Pergamon Press, Oxford, pp 351–468. doi:10.1016/B978-008096518-5.00111-8
Yavari I, Ramazani A (1996) Vinyltriphenylphosphonium salt mediated preparation of dialkyl 2H-1-benzopyran-2,3-dicarboxylates. An efficient one-pot synthesis of 2H-chromene derivatives. J Chem Res Synop 8: 382–383
Lindell SD (1995) Stabilized substituted ions and radicals bearing one heteroatom (R1R2C?X, R1R2C+X, R1R2C*X) In: Katritzky AR, Meth-Cohn O, Rees CW (eds) Comprehensive organic functional group transformations. Pergamon, New York, vol 2. pp 997–1010. doi:10.1016/B0-08-044705-8/00261-2
Olah GA, Calin A (1968) Protonation of halogen-containing benzenes in Hf-SbF5, I, Proton magnetic resonance spectra of fluoro-chloro-, and bromoesitylenium and xylenium ions. J Am Chem Soc 90: 938. doi:10.1021/ja01006a017
Childs RF, Faggiani R, Lock CJL, Mahendran M, Zweep SD (1986) Structures of two cyclopropylcarbinyl cations. J Am Chem Soc 108: 1692–1693. doi:10.1021/ja00267a050
Mir-Mohamad-Sadeghy M, Rickborn B (1983) Benzo[f]isobenzofuran. Mechanistic aspects of isobenzofuran formation from acetals and ortho esters. J Org Chem 48: 2237. doi:10.1021/jo00161a020
Cornejo JJ, Ghodsi S, Johnson RD, Woodling R, Rickborn B (1983) Benzo[e]isobenzofuran. Formation and reactions of the parent and alkoxy-substituted derivatives. J Org Chem 48: 3869. doi:10.1021/jo00170a001
Ohmura H, Mikami K (2001) Heterogeneous acid-catalyzed (2,5) oxoniumene reaction for eight-membered ring formation. Tetrahedron Lett 42: 6859–6863. doi:10.1016/S0040-4039(01)01363-6
Olah GA, Svoboda JJ (1972) Preparative carbocation chemistry. III, improved preparation of alkyloxocarbenium (Acyl) hexafluoroantimonate salts. Synthesis-Stuttgart 6: 306. doi:10.1055/s-1972-21872
Joule JA, Mills K, Smith GF (1995) Heterocyclic Chemistry. 3. Chapman and Hall, New York, pp 146–183
Fichtner C, Remennikov G, Mayr H (2001) Kinetics of the reactions of flavylium ions with nucleophiles. Eur J Org Chem 2001((23): 4451–4456. doi:10.1002/1099-0690(200112)2001:23<4451::AID-EJOC4451>3.0.CO;2-F
Lu Y, Foo LY (2002) Unexpected rearrangement of pyranoanthocyanidins to furoanthocyanidins. Tetrahedron Lett 43: 715–718. doi:10.1016/S0040-4039(01)02236-5
Basavaiah D, Sreenivasulu B, Rao JS (2001) A novel Baylis– Hillman protocol for the synthesis of functionalized fused furans. Tetrahedron Lett 42: 1147–1149. doi:10.1016/S0040-4039(00)02175-4
Cave GWV, Raston CL, Scott JL (2001) Recent advances in solventless organic reactions: towards benign synthesis with remarkable versatility. Chem Commun 2159–2169. doi:10.1039/b106677n
Sheldon RA (1997) As estimated by determination of E-factor. Catalysis and pollution prevention. Chem Ind 12–15
Tebby JC, Verkede JG, Quin LD (1987) Phosphorus-31 NMR spectroscopy in stereochemical analysis, chap 1. VCH Publishers, Weinheim, p 34
Skattebol L, Jones ERH, Whiting MC (1963) 1-Phenyl-1-penten-4-yn-3-ol [1-penten-4-yn-3-ol, 1-phenyl-]. Org Synth Coll 4: 792
Bowden K, Heilbron IM, Jones ERH, Weedon BC (1946)Researches on acetylenic compounds Pan I. The preparation of acetylenic ketones by oxidation of acetylenic carbinols and glycols. J Chem Soc (London) 1946: 39–45
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalilzadeh, M.A., Hossaini, Z., Charati, F.R. et al. A mild and efficient method for the synthesis of a new class of furo[3,2-c]chromenes in aqueous media. Mol Divers 15, 445–450 (2011). https://doi.org/10.1007/s11030-010-9264-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-010-9264-3