Abstract
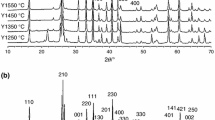
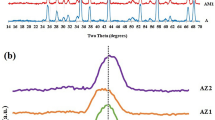
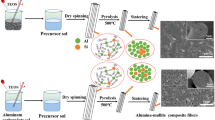
This paper compares the microstructure development of two alumina–15 vol% mullite composites during the sintering. The nanopowders of alumina–mullite composite were synthesized by the sol–gel method using aluminum chloride hexahydrate and two different silicon sources (colloidal silica in route 1 and tetraethyl orthosilicate in route 2). The alumina–mullite composites were prepared by pressing and sintering of the nanopowders. Although the intergranular mullites were observed in both routes, there were mullite particles in route 2 formed inside the alumina grains (intragranular mullite). Formation of the intragranular mullite is attributed to the drop in silica solubility, which occurs during the transition from metastable alumina to stable alumina. Compared to route 1, the relative density and the average grain size were increased and accelerated by route 2. The two-stage sintering is not useful for the mullite decomposition.








Similar content being viewed by others
Notes
Orthorhombic [4]. a = 7.54 ± 0.03 Å; b = 7.693 ± 0.03 Å; c = 2.890 ± 0.01.
Merck 101084.
Sigma–Aldrich 4208404 (40 wt.% suspension in H2O).
Sigma–Aldrich 131903.
References
Richerson DW (1992) Modern ceramic engineering. Marcel Dekker, New York, pp 808–823
Medvedovski E (2006) Alumina–mullite ceramics for structural applications. Ceram Int 32:369–375
Luo HH, Zhang FC, Roberts SG (2008) Wear resistance of reaction sintered alumina/mullite composites. Mat Sci Eng A 478:270–275
Shackelford JF, Alexander W (2001) Materials science and engineering handbook. CRC Press LLC, USA
Davis RF, Pask JA (1972) Diffusion and reaction studies in the system Al2O3–SiO2. J Am Ceram Soc 55:525–531
Aksaf IA, Pask JA (1975) Stable and metastable equilibria in the system SiO2–Al2O3. J Am Ceram Soc 58:507–512
Aksay IA, Dabbs DM, Sarikaya M (1991) Mullite for structural, electronic, and optical applications. J Am Ceram Soc 74:2343–2358
Mezquita S, Uribe R, Moreno R, Baudín C (2001) Influence of mullite additions on thermal shock resistance of dense alumina materials, Part 2: Thermal properties and thermal shock behaviour. Br Ceram Trans 100(6):246–250
Aksel C (2003) The effect of mullite on the mechanical properties and thermal shock behaviour of alumina–mullite refractory materials. Ceram Int 29:183–188
Zhang FC, Luo HH, Roberts SG (2007) Mechanical properties and microstructure of Al2O3/mullite composite. J Mater Sci 42:6798–6802
Aksel C (2002) The role of fine alumina and mullite particles on the thermomechanical behaviour of alumina–mullite refractory materials. Mater Lett 57:708–714
Moreno R, Mezquita S, Baudín C (2001) Influence of mullite additions on thermal shock resistance of dense alumina materials, Part 1: Processing studies. Br Ceram Trans 100(6):241–245
Thompson AM, Soni KK, Chan HM, Harmer MP, Williams DB, Chabala JM, Levi-Scotti R (1997) Dopant distributions in rare-earth-doped alumina. J Am Ceram Soc 80(2):373–376
Fang J, Thompson AM, Harmer MP, Chan HM (1997) Effect of yttrium and lanthanum on the final-stage sintering behavior of ultrahigh-purity alumina. J Am Ceram Soc 80(8):2005–2012
Schehl M, Díaz LA, Torrecilla R (2002) Alumina nanocomposites from powder-alkoxide mixtures. Acta Mater 50:1125–1139
Won CW, Siffert B (1998) Preparation by sol–gel method of SiO2 and mullite (3Al203·2SiO2) powders and study of their surface characteristics by inverse gas chromatography and zetametry. Colloid Surf A Physicochem Eng Asp 131:161–172
Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products, ASTM Designation (2005) :C 373–388, ASTM standards, vol. 15.02
Powder Diffraction File, Card No. 10-0425, JCPDS
Powder Diffraction File, Card No. 04-0878, JCPDS
Powder Diffraction File, Card No. 01-087-2096, JCPDS
Powder Diffraction File, Card No. 01-085-0419, JCPDS
Sedaghat A, Taheri-Nassaj E, Naghizadeh R (2006) An alumina mat with a nano microstructure prepared by centrifugal spinning method. J Non-cryst Solids 352:2818–2828
Powder Diffraction File, Card No. 15-0776, JCPDS
Powder Diffraction File, Card No. 10-0173, JCPDS
Jacobson NS (1993) Corrosion of silicon-based ceramics in combustion environments. J Am Ceram Soc 76(1):3–28
Opila EJ (2003) Oxidation and volatilization of silica formers in water vapor. J Am Ceram Soc 86(8):1238–1248
Hildmann B, Braue W, Schneider H (2008) Topotactic growth of α-alumina platelets on 2/1 mullite single crystal surfaces upon thermal decomposition of mullite in dry and wet atmospheres. J Eur Ceram Soc 28:407–423
Schneider H, Komarneni S (2005) Mullite. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 236–237
Okada K, Otsuka N, Sh Somiya (1991) Review of mullite synthesis routes in Japan. Am Ceram Soc Bull 70(10):1633–1640
Huling JF, Messing GL (1992) Chemistry-crystallization relations in molecular mullite gels. J Non-Cryst Solids 147,148: 213–221
Imose M, Takano Y, Yoshinaka M, Hirota K, Yamaguchi O (1998) Novel synthesis of mullite powder with high surface area. J Am Ceram Soc 81(6):1537–1540
Bowen P, Carry C, Luxembourg D, Hofmann H (2005) Colloidal processing and sintering of nanosized transition aluminas. Powder Technol 157:100–107
Bodišová K, Šajgalík P, Galusek D, Švančárek P (2007) Two-stage sintering of alumina with submicrometer grain size. J Am Ceram Soc 90(1):330–332
Li J, Ye Y (2006) Densification and grain growth of Al2O3 nanoceramics during pressureless sintering. J Am Ceram Soc 89(1):139–143
Kanters J, Eisele U, Rödel J (2000) Effect of initial grain size on sintering trajectories. Acta Mater 48:1239–1246
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sedaghat, A., Taheri-Nassaj, E., Soraru, G.D. et al. A comparative study of microstructural development in the sol–gel derived alumina–mullite nanocomposites using colloidal silica and tetraethyl orthosilicate. J Sol-Gel Sci Technol 58, 689–697 (2011). https://doi.org/10.1007/s10971-011-2446-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2446-3