Abstract
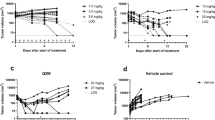
Dulanermin (rhApo2L/TRAIL) and conatumumab bind to transmembrane death receptors and trigger the extrinsic cellular apoptotic pathway through a caspase-signaling cascade resulting in cell death. Tumor size time series data from rodent tumor xenograft (COLO205) studies following administration of either of these two pro-apoptotic receptor agonists (PARAs) were combined to develop a intracellular-signaling tumor-regression model that includes two levels of signaling: upstream signals unique to each compound (representing initiator caspases), and a common downstream apoptosis signal (representing executioner caspases) shared by the two agents. Pharmacokinetic (PK) models for each drug were developed based on plasma concentration data following intravenous and/or intraperitoneal administration of the compounds and were used in the subsequent intracellular-signaling tumor-regression modeling. A model relating the PK of the two PARAs to their respective and common downstream signals, and to the resulting tumor burden was developed using mouse xenograft tumor size measurements from 448 experiments that included a wide range of dose sizes and dosing schedules. Incorporation of a pro-survival signal—consistent with the hypothesis that PARAs may also result in the upregulation of pro-survival factors that can lead to a reduction in effectiveness of PARAs with treatment—resulted in improved predictions of tumor volume data, especially for data from the long-term dosing experiments.



Similar content being viewed by others
References
Ashkenazi A (2008) Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov 7:1001–1012
Bouralexis S, Findlay DM, Evdokiou A (2005) Death to the bad guys: targeting cancer via Apo2L/TRAIL. Apoptosis 10:35–51
Weinberg RA (2007) The biology of cancer. Garland Science, New York
Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS, Novotny W, Goldwasser MA, Tohnya TM, Lum BL, Ashkenazi A, Jubb AM, Mendelson DS (2010) Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol 28:2839–2846
Bajaj M, Heath EI (2011) Conatumumab: a novel monoclonal antibody against death receptor 5 for the treatment of advanced malignancies in adults. Expert Opin Biol Ther 11:1519–1524
Holland PM (2011) Targeting Apo2L/TRAIL receptors by soluble Apo2L/TRAIL. Cancer Lett. doi:10.1016/j.canlet.2010.11.001
Sharma S, de Vries E, Infante J, Oldenhuis C, Chiang L, Bilic S, Goldbrunner M, Scott J, Burris H (2008) Phase I trial of LBY135, a monoclonal antibody agonist to DR5, alone and in combination with capecitabine in advanced solid tumors. J Clin Oncol 26:3538
Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S (2010) Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer 102:506–512
Sikic B, Wakelee H, von Mehren M, Lewis N, Calvert A, Plummer E, Fox N, Howard T, Jones S, Burris H (2007) A phase Ib study to assess the safety of lexatumumab, a human monoclonal antibody that activates TRAIL-R2, in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI. J Clin Oncol 25:14006
Almasan A, Ashkenazi A (2003) Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev 14:337–348
LeBlanc HN, Ashkenazi A (2003) Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ 10:66–75
Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, Moriguchi J, Coxon A, Huard JN, Xu R, Peach ML, Juan G, Kaufman S, Chen Q, Bianchi A, Kordich JJ, Ma M, Foltz IN, Gliniak BC (2010) Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol Ther 9:618–631
Albeck JG, Burke JM, Aldridge BB, Zhang M, Lauffenburger DA, Sorger PK (2008) Quantitative analysis of pathways controlling extrinsic apoptosis in single cells. Mol Cell 30:11–25
Fussenegger M, Bailey JE, Varner J (2000) A mathematical model of caspase function in apoptosis. Nat Biotechnol 18:768–774
Harrold J, Straubinger RM, Mager D (2012) Combinatorial chemotherapeutic efficacy in non-Hodgkin lymphoma can be predicted by a signaling model of CD20 pharmacodynamics. Cancer Res 72:1632–1641
Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 299:31–38
Bruno R, Lu JF, Sun N, Claret L (2011) A modeling and simulation framework to support early clinical drug development decisions in oncology. J Clin Pharmacol 51:6–8
Simeoni M, Magni P, Cammia C, De Nicolao G, Croci V, Pesenti E, Germani M, Poggesi I, Rocchetti M (2004) Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res 64:1094–1101
Yang J, Mager DE, Straubinger RM (2010) Comparison of two pharmacodynamic transduction models for the analysis of tumor therapeutic responses in model systems. AAPS J 12:1–10
Yamazaki S, Skaptason J, Romero D, Lee JH, Zou HY, Christensen JG, Koup JR, Smith BJ, Koudriakova T (2008) Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug Metab Dispos 36:1267–1274
D’Argenio DZ, Schumitzky A, Wang X (2009) ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles
Schumitzky A (1995) EM Algorithms and two stage methods in pharmacokinetic population analysis. In: D’Argenio DZ (ed) Advanced methods of pharmacokinetic and pharmacodynamic systems analysis, vol 2. Plenum Press, New York, pp 140–160
Walker S (1996) An EM algorithm for nonlinear random effects models. Biometrics 52:934–944
Bauer RJ, Guzy S (2004) Monte Carlo parameter expectation maximization (MC-PEM) method for analyzing population pharmacokinetic/pharmacodynamic data. In: D’Argenio DZ (ed) Advanced methods of pharmacokinetic and pharmacodynamic systems analysis, vol 3. Kluwer Academic Publishers, Boston, pp 155–163
Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L (1997) Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7:821–830
Hsu H, Shu HB, Pan MG, Goeddel DV (1996) TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299–308
Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J (1997) TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 7:831–836
Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J (2001) NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 21:5299–5305
Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol 3:401–410
Choi YE, Butterworth M, Malladi S, Duckett CS, Cohen GM, Bratton SB (2009) The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J Biol Chem 284:12772–12782
Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A (2006) Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst 98:335–344
Curnis F, Sacchi A, Corti A (2002) Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest 110:475–482
Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E (2010) Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328:1031–1035
Jain RK (1994) Barriers to drug delivery in solid tumors. Sci Am 271:58–65
Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer 6:583–592
Albeck JG, Burke JM, Spencer SL, Lauffenburger DA, Sorger PK (2008) Modeling a snap-action, variable-delay switch controlling extrinsic cell death. PLoS Biol 6:2831–2852
Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM (2001) Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol 19:1501–1518
Daniel D, Yang B, Lawrence DA, Totpal K, Balter I, Lee WP, Gogineni A, Cole MJ, Yee SF, Ross S, Ashkenazi A (2007) Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood 110:4037–4046
Acknowledgments
We gratefully acknowledge the helpful comments provided by Liviawati Sutjandra, an employee of Amgen Inc. We would also like to thank Michelle Zakson, an employee of Amgen Inc, for providing editorial and formatting assistance with the manuscript. This work was supported by Amgen Inc., as well as by grant NIH/NIBIB P41-EB001978 (DZD).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A
This appendix includes the details of the dulanermin and conatumumab treatment groups, with the doses, regimens, and number of animals listed. Table 3 describes the individual dulanermin data, Table 4 describes the average dulanermin data, and Table 5 describes the individual conatumumab data.
Appendix B
This appendix includes the details of the dulanermin and conatumumab PK models. Model equations and parameter estimates are provided for both compounds.
A linear two-compartment model described the mean dulanermin IV plasma concentration data (only mean data available). Given these estimates for the disposition parameters, the mean data available following IP administration of dulanermin were described using an absorption model with separate parallel slow and rapid absorption components. The complete pharmacokinetic model for dulanermin is as follows:
The variables and parameters are defined as follows, along with the values of the model parameter estimates: x 1 is the amount of dulanermin in the plasma compartment; x 2 is the amount of drug in the peripheral compartment; x 3 is the amount of drug in the rapid IP-delivery compartment; x 4 is the amount of drug in the first slow IP-delivery compartment; x 5 is the amount of drug in the second slow IP-delivery compartment; k 10,Dlmn is the plasma elimination constant of dulanermin (232/day); k 12,Dlmn (3.76/day)and k 21,Dlmn (10.8/day) are the rate constants between the plasma compartment and the periphery; k a,Dlmn (18.9/day) is the rate constant for rapid IP delivery; k aa,Dlmn (19.6/day) is the rate constant for slow IP delivery; F 1 (0.242) is the IP fraction that is delivered to the first slow IP-delivery compartment; F 2 (0.097) is the IP fraction that is delivered to the rapid IP-delivery compartment; Dose IV and Dose IP represent the IV and IP dosing regimens defined in Appendix A; and V 1 (48.6 mL/kg) is the plasma volume of distribution. (F Total (0.339) is the sum of F 1 and F 2, and is the total fraction of the IP dose absorbed.) The initial condition of each state is fixed at zero. See Fig. 4 for model fits to the dulanermin plasma concentration data following IV (left panel) and IP (right panel) administration.
A linear two-compartment model was fit to all the median conatumumab plasma concentration data from all the experiments (naïve pooled data analysis). The model parameters and maximum likelihood estimates are given as follows: elimination rate constant, k 10,Cmab = 0.0913/day; central to peripheral rate constant, k 12,Cmab = 0.251/day; peripheral to central rate constant, k 21,Cmab = 0.403/day; intraperitoneal absorption rate into central compartment, k a,Cmab = 8.17/day; Dose IP represents the IP dosing regimens defined in Appendix A; ratio of plasma volume of distribution to fraction of conatumumab absorbed IP (which cannot be distinguished because no separate IV data is available), V/F = 2.99 mL. The initial condition of each state is fixed at zero. See Fig. 5 for selected model fit plots to the conatumumab plasma concentration data following IP administration.
Sample model fits (2 of 13 experiments shown) to median plasma concentration data for conatumumab given IP (left panel: single dose of 240 mg; right panel: 36.9 mg given twice weekly for 4 weeks). The solid circles represent the median of the measured data at each time point; the dashed lines represent the estimated model predictions
Rights and permissions
About this article
Cite this article
Kay, B.P., Hsu, CP., Lu, JF. et al. Intracellular-signaling tumor-regression modeling of the pro-apoptotic receptor agonists dulanermin and conatumumab. J Pharmacokinet Pharmacodyn 39, 577–590 (2012). https://doi.org/10.1007/s10928-012-9269-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9269-x