Abstract
Sthenurine kangaroos, extinct “giant kangaroos” known predominantly from the Plio-Pleistocene, have been proposed to have used bipedal striding as a mode of locomotion, based on the morphology of their hind limbs. However, sthenurine forelimb morphology has not been considered in this context, and has important bearing as to whether these kangaroos employed quadrupedal or pentapedal locomotion as a slow gait, as in extant kangaroos. Study of the correlation of morphology of the proximal humerus in a broad range of therian mammals shows that humeral morphology is indicative of the degree of weight-bearing on the forelimbs during locomotion, with terrestrial species being distinctly different from arboreal ones. Extant kangaroos have a proximal humeral morphology similar to extant scansorial (semi-arboreal) mammals, but sthenurine humeri resemble those of suspensory arboreal taxa, which rarely bear weight on their forelimbs, supporting the hypothesis that they used bipedal striding rather than quadrupedal locomotion at slow gaits. The humeral morphology of the enigmatic extinct “giant wallaby,” Protemnodon, may be indicative of a greater extent of quadrupedal locomotion than in extant kangaroos.
Similar content being viewed by others
Introduction
The familiar locomotor mode of kangaroos is hopping: that is, bipedal locomotion using the hind limbs in tandem, unaided by the forelimbs. But, at the slower speeds that make up the majority of the daily locomotor repertoire of kangaroos, some sort of forelimb-supported gait is employed, and this locomotion dominates the pattern of daily movement (see Doube et al. 2018). In the Pleistocene there was a diversity of kangaroo species much larger than the extant ones, the so-called “giant kangaroos.” These include the giant short-faced browsing kangaroos (subfamily Sthenurinae; family Macropodidae), and the so-called “giant wallaby,” Protemnodon (subfamily Macropodinae; family Macropodidae). Here, we consider the mode of slow locomotion in these large extinct kangaroos by an examination of the morphology of their proximal humeri in comparison with extant mammals that exhibit differing degrees of weight-bearing on their forelimbs during locomotion.
Sthenurines are first known from the middle Miocene, and were always relatively large animals for their time, although truly “giant” forms (i.e., larger than extant kangaroos) are not known until the Plio-Pleistocene. Miocene sthenurines include the late middle to early late Miocene Wanburoo (Kear et al. 2001; body mass ~7–8 kg, Travouillon et al. 2009); the early late Miocene Rhizosthenurus (see Kear 2002; body mass ~9–15 kg, Travouillon et al. 2009); and the late late Miocene Hadronomas (see Murray 1991, of similar size to a modern large grey kangaroo, i.e., ~50 kg). Plio-Pleistocene sthenurines comprised three to five genera and ~25 species (Prideaux 2004), with body mass estimates of 43–244 kg (Helgen et al. 2006). Species (~10) of Protemnodon are known only from the Plio-Pleistocene, with body mass estimates of 43–166 kg (Helgen et al. 2006). Ancient DNA has shown that Protemnodon is quite closely related to the genus Macropus (Llamas et al. 2015). In contrast, the extant large species of Macropus have body masses of between around 25 kg (small female M. giganteus) and 80 kg (large male M. rufus) (Dawson 1995); but in the Pleistocene larger species existed (M. titan and M. ferragus) with body masses of up to 180 kg (Helgen et al. 2006).
Kangaroo Locomotion: Speed and Size Constraints
Hopping is an extremely efficient fast gait, with a relatively flat relationship between speed and energetic costs in larger kangaroos (Baudinette 1989; Bennett and Taylor 1995). All extant macropodoids (kangaroos and rat-kangaroos), with the exception of the musky rat-kangaroo, Hypsiprymnodon moschatus (Burk et al. 1998), engage in at least some hopping. There appear to be size limits on the use of hopping locomotion. Hopping mammals do not alter their locomotor posture with increasing body size; larger hoppers maintain the same crouched limb posture as smaller ones, which results in increasing tendon strain in the hind legs of larger kangaroos (McGowan et al. 2008; Snelling et al. 2017). The optimal size for hopping has been estimated at around 50 kg (which is around the average body weight of large extant kangaroos) (Bennett and Taylor 1995). A maximum possible size, beyond which tendon safety factors would be below unity, has been estimated at ~140 kg (McGowan et al. 2008).
Hopping is biomechanically impossible at slow speeds (Baudinette 1989), necessitating the use of an alternative gait. At speeds below around 6 km/h all extant kangaroos perform some sort of gait that involves bearing weight on both forelimbs and hind limbs, either walking or bounding (Windsor and Dagg 1971). The specialized slow gait of extant large kangaroos is a pentapedal walk, with the tail acting as a “fifth limb” to aid in propulsion (O’Connor et al. 2014; Dawson et al. 2015). In contrast with hopping locomotion, the energetic costs of quadrupedal bounding increase with speed, and scale in a similar fashion to the costs of quadrupedal locomotion in other mammals. Pentapedal locomotion appears to be more expensive than quadrupedal bounding (Bennett 2000), but is probably mandated for large kangaroos with extremely long hind legs (Dawson et al. 2015).
Morphology of Extinct “Giant Kangaroos”, and Proposed Modes of Locomotion
The skeleton of sthenurines was more robust than that of extant large kangaroos, and their body size was not only above the theoretical optimum size for hopping (~50 kg; Bennett and Taylor 1995) but, for several species, above the theoretical maximum size for hopping (140 kg) (Helgen et al. 2006; McGowan et al. 2008). Janis et al. (2014), in a study of hind limb morphology, proposed that sthenurines used bipedal striding as their main form of locomotion, although most probably did some hopping: only Procoptodon goliah (estimated body mass of 232 kg; Helgen et al. 2006) was significantly above the proposed maximum size for hopping locomotion, but Sthenurus stirlingi (included in this study, estimated body mass of 173 kg; Helgen et al. 2006) may also have been at or above the theoretical limit.
Janis et al. (2014) proposed that sthenurines would have especially employed a bipedal striding gait at slow speeds instead of quadrupedal (or pentapedal) walking, a gait that would likely have been impossible for these kangaroos. Sthenurines had a stiffly-braced lumbar vertebral region, with limited capacity for dorso-ventral flexion, and specialized long-fingered hands that had limited capacity for dorsiflexion (Wells and Tedford 1995). This anatomy is interpreted as an adaptation for browsing with an upright bipedal posture, and likely would have prevented sthenurines from employing the quadrupedal or pentapedal gaits of extant kangaroos (Wells and Tedford 1995). However, Janis et al. (2014) did not examine any anatomical indicators that would support the hypothesis that sthenurines did less weight-bearing on the forelimbs than extant kangaroos.
Some Protemnodon species were also above the theoretical size limit for hopping (e.g., Protemnodon roechus, body mass estimate of 166 kg; Helgen et al. 2006). While the species of Protemnodon included here, Protemnodon anak and Protemnodon brehus (estimated body masses of 131 kg and 110 kg, respectively; Helgen et al. 2006) were not above the theoretical maximum size, they were well above the optimal size of ~50 kg. Protemnodon species also had skeletons that appear robust in comparison with large species of Macropus (Janis et al. 2014). The skeleton of Protemnodon does not appear well adapted for hopping, with short metatarsals in comparison to other large kangaroos, and it has been suggested that at least some species were quadrupedal at all gaits (Kear et al. 2008; Den Boer 2018). Den Boer (2018) considered the limb proportions of Protemnodon anak, but did not specifically consider the capacity for weight-bearing on the forelimbs; if this animal was indeed primarily quadrupedal, then one would predict that it would have morphology evidencing better capacity for this behavior than extant kangaroos.
Proximal Humeral Morphology and Forelimb Support
The major features of the therian (placental and marsupial) mammalian proximal humerus are the articular head (articulating with the scapula glenoid); the greater tuberosity (= greater tubercle), which serves as the insertion point for the supra- and infraspinatus muscles and the teres minor muscle; the lesser tuberosity (= lesser tubercle), which serves as the insertion point for the subscapularis muscle; and the intertubercle sulcus (= the bicipital groove), which is the passageway for the tendon of the biceps brachii. The muscles attaching to the proximal humerus are known as the rotator cuff muscles in humans, where they act to protract, rotate, adduct, and abduct the humerus, as well as stabilizing the glenohumeral joint (Mathewson et al. 2014).
The morphology of the proximal humerus is indicative of the extent of weight-bearing on the forelimbs during locomotion. Joint morphology is a compromise between mobility and stability, and this is especially important in the case of the therian mammal shoulder joint. Therians are unusual among tetrapods in having a humero-scapular joint that has a very shallow ball-and-socket articulation. Such morphology potentially allows for great mobility of the arm, but the therian shoulder joint must now be stabilized by muscular action (Jenkins and Weijs 1979).
Terrestrial therians, habitually bearing weight on their forelimbs, have a very different morphology of the proximal humerus from arboreal ones, where rotational capacities of the humerus are more important than stability in bearing weight during locomotion. In accordance, as will be explained below in functional terms: arboreal mammals have a rounded, globular humeral head, with relatively small tuberosities that do not project above the surface of the head; terrestrial mammals have a more ovoid (elliptical), flatter humeral head, with relatively larger tuberosities (especially the greater tuberosity) that project above the level of the humeral head (see Fig. 1).
Examples of proximal humeri of extant mammals. a. Arboreal, red panda (Ailurus fulgens, based on MCZ 64643). b. Suspensory, spider monkey (Ateles paniscus, based on AMNH 35709). c. Scansorial, narrow-striped mongoose (Mungotictis decemlineata, based on FMNH 176128). d. Terrestrial, fanaloka (Fossa fossana, based on FMNH 85196)
These differences in humeral proximal morphology between terrestrial and arboreal forms have primarily been observed in primates (e.g., Szalay and Dagosto 1980; Rose 1989; Gebo and Sargis 1994; Ride et al. 1997; Schmitt 2003; Arias-Martorell 2018), but also in other mammals such as tree shrews (Sargis 2002), tenrecs (Salton and Sargis 2008), didelphid marsupials (Argot 2001; Szalay and Sargis 2001), viverrids (Taylor 1974), felids (Walmesly et al. 2012), procyonids (Tarquini et al. 2019), caviomorph rodents (Morgan and Álverez 2013), and xenarthrans (Toledo et al. 2013). Although most authors reported a phylogenetic signal within these lineages, the overall pattern of similarities both within and between lineages, and in marsupials as well as placentals, evidences a strong functional association between morphology and locomotion that overrides any phylogenetic effects.
The supraspinatus and infraspinatus muscles are often considered to act as protractors of the humerus, but electromyographic studies show that they are primarily active while the foot is on the ground in the stance phase, rather than while the foot is moving forwards in the swing phase, and they thus act primarily as stabilizers. This pattern of muscular activity is seen both in primates (Larson and Stern 1989) and didelphid marsupials (Jenkins and Weijs 1979), so thus is likely the basal role of these muscles in therian mammals. Larger mammals such as ungulates have the infraspinatus muscle as the predominant rotator cuff muscle, while in primates and smaller mammals such as rodents and rabbits the subscapularis is the predominant muscle (Mathewson et al. 2014): this would thus also appear to be the basal mammalian condition. In a “true,” human-like, rotator cuff, the rotator cuff muscles – supraspinatus, infraspinatus, and teres minor – combine into a single tendon for their insertion onto the greater tuberosity. Such an anatomical set-up is seen only in hominoid primates among placentals, and only in tree-kangaroos among a diversity of marsupials, and is likely functionally related to the ability to raise the arm above the head (Sonnabend and Young 2009). This may also have been the case in sthenurine kangaroos.
The joint-stabilizing function of the rotator cuff muscles is critical in mammals that bear weight on their forelimbs. In predominantly terrestrial mammals the humerus is primarily limited to parasagittal motion, and the infraspinatus and teres minor are especially important in stabilizing the glenohumeral joint against adduction (Mathewson et al. 2014). The more ovoid humeral head of terrestrial mammals restricts the motion of the humerus to the parasagittal plane, and the large greater tuberosity restricts humeral abduction as well as acting as an increased lever arm for the supraspinatus and infraspinatus muscles. In contrast, the rounded, globular humeral head in arboreal mammals allows for greater rotational ability of the humerus in the socket, and the lower relief of the tuberosities provides a greater area of attachment for the rotator cuff muscles around the humeral head (Gebo and Sargis 1994).
Although all terrestrial therians have an enlarged greater tuberosity, in many lineages the size of the lesser tuberosity varies little with locomotor mode. However, there are some exceptions. In suspensory (under-branch hanging) hominoids, as opposed to merely arboreal ones (which are usually branch-walkers), the lesser tuberosity is greatly reduced in size, which may allow for an even greater rotational ability of the humerus on the scapula (Arias-Martorell 2018). In contrast, some arboreal mammals that use their forelimbs in active climbing (rather than for suspension) have an enlarged, medially-directed lesser tuberosity: this is seen in certain tree shrews (Sargis 2002), didelphids (Argot 2001), carnivorans (Gebo and Rose 1993), and also in the suspensory sloths (Toledo et al. 2013). An enlarged lesser tuberosity is also observable in our photographs in lemuriform primates such as the indri (Indri indri) and the diademed sifaka (Propithecus diadema), the giant pangolin (Smutsia gigantea), both sloths (Bradypus variagata and Coelepus hofmanni), the fairy anteater (Cyclopes diadactylus), and the tamandua (Tamandua tetradactyla). This morphology of the lesser tuberosity provides a greater moment arm for the action of the subscapularis muscle, which is important in the medial (internal) rotation of the humerus (Sargis 2002; Arias-Martorell 2018).
Materials and Methods
Two different modes of data analyses were performed to investigate the correlation of proximal humeral shape with locomotor mode: one using 2D Geometric Morphometrics, and one using areas of portions of the humerus analyzed in a phylogenetic framework. Our motivation for the second analysis was as follows. Our interest is in the functional morphology of the proximal humerus rather than its precise anatomy: we thus propose that the relative sizes of the greater tuberosity, lesser tuberosity, and humeral head are a direct proxy of humeral function. In general terms, differences in the relative sizes of these structures between arboreal and terrestrial taxa should apply across taxonomic groups. However, geometric morphometrics records not only the relative size of these elements, but also aspects of shape, such as the relative positioning of the tuberosities with respect to the humeral head, and the precise shape of the tuberosities themselves (the greater tuberosity, in particular, can be highly variable in shape). Thus any analysis to determine phylogenetic effects could be confounded by an effect of phylogeny with respect to these other areas of shape, rather than on the relative sizes of the humeral components alone. We controlled for the possible influence of these shape factors by conducting this second analysis only on the relative size of the three different areas of the proximal humeral anatomy (i.e., the humeral head and the two tuberosities), and here we controlled for potential effects of phylogenetic nonindependence to completely isolate the functional signal (relative size) from confounding influences.
Data Collection
The humeri of 115 mammalian species were photographed in superior view, such that the humeral head and greater and lesser tuberosities were visible in what would be the plane of articulation with the scapula (see Fig. 1). The photographs of left humeri were mirror-imaged so that the data analyzed included only effectively right-side humeri.
The species included comprised five species of extinct kangaroos, 13 extant macropodoid species (kangaroos and rat-kangaroos, families Macropodidae and Potoroidae, respectively); nine species of other extant (or recently extinct) marsupials (families Dasyuridae, Didelphidae, Peramelidae, Phalangeriidae, Phascolarctidae, Thylacinidae, and Vombatidae); and 87 species of extant placentals (orders Carnivora, Cingulata, Pholidota, Pilosa, Primates, Rodentia) (see Table 1).
The extinct kangaroos included: subfamily Sthenurinae – “Procoptodon” gilli (~54 kg), “Procoptodon” browneorum (similar size to “Procoptodon” gilli), two individuals of Simosthenurus occidentalis (~118 kg), and two individuals of Sthenurus stirlingi (~173 kg); subfamily Macropodinae –Protemnodon anak (~131 kg) and Protemnodon brehus (~110 kg). All body mass estimates are from Helgen et al. (2006).
The selection of extant mammals represented those within the size range of extant and extinct kangaroos, ranging from ~1 kg to ~150 kg, including lineages that comprised a diversity of locomotor types (see below). An average body mass for each taxon was taken from the PanTHERIA database (Jones et al. 2009). Some phalangerid marsupials and sciurid rodents were slightly smaller (but all above 500 g), and were included in order to obtain a balance of locomotor types. (See Table 1.)
The extant mammals were assigned to the following locomotor modes (from information in a diversity of literature sources): arboreal (primarily living in trees, rarely locomoting on the ground, 24 taxa); terrestrial (primarily living on the ground, almost never climbing trees, 37 taxa); scansorial (= semi-arboreal, locomoting both on the ground and within the canopy, 27 taxa); and ricochetal (hopping, macropodoids plus the rodent Pedetes capensis, 13 taxa). A separate category of arboreal forms included mammals with suspensory types of locomotion (from the orders Pilosa and Primates, eight taxa). We acknowledge that these categories, especially the distinction between arboreal and scansorial, may not be completely distinct, but we employ them in the spirit of the notion of locomotor mode morphotype (see Szalay and Dagosto 1980). Because of the paucity of extant larger species of marsupials, all available taxa were included. In the case of placentals, the selected lineages mainly represent orders, and usually only one species per genus was included. Taxa from the orders Artiodactyla, Lagomorpha, and Macroscelidea, and Hyracoidea were not included. The former three orders had a diversity of species within the determined size range, but all were terrestrial. Although Hyracoidea includes a diversity of locomotor types, all hyrax species have an extremely large humeral greater tuberosity, and we decided not to include them.
Specimens were photographed in the collections of a diversity of institutions (see legend for Table 1). All specimens were photographed with a scale bar for later use in digital measurement. Most specimens were photographed with a Nikon DSLR camera or a FujiFilm FinePix S9900 W; however, the smallest were photographed with a Celestron Digital Microscope Pro connected to a MacBook Air.
Data availability. The measurements (for the proximal humerus of each taxon) are available, in the form of the Procrustes Coordinates (for the Geometric Morphometric data: Online Resource 1), and the raw and relative area measurements (for the Area Measurements: Online Resource 2, which also includes the first and last occurrences for each taxon).
Geometric Morphometrics
The photographs of the proximal humerus were scaled and a series of ten landmarks were digitized on each one (Fig. 2a). These landmarks were selected to capture the main features of the shape of the proximal humeral epiphysis, including the head, the greater and lesser tuberosities, and the bicipital groove. This process was developed using the TPS Util 1.68 and TPS Dig 2.25 (Rohlf 2016a, b). These raw landmark coordinates were imported onto the software MorphoJ (Klingenberg 2011). A Procrustes alignment was performed to remove the differences in size, translation, and rotation (Dryden and Mardia 1998). The resulting shape (Procrustes) coordinates were imported to R environment (RStudio Team 2015) and the effect of allometry was explored within a phylogenetic context (see below for phylogenetic tree composition) using procD.pgls function of geomorph package (Adams and Otárola-Castillo 2013). In addition, the phylogenetic signal was also tested using physignal function of the same package (Adams and Otárola-Castillo 2013). Following this, these Procrustes coordinates were used to carry out a principal components analysis (PCA) and a canonical variates analysis (CVA) in MorphoJ (Klingenberg 2011).
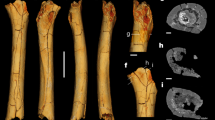
Proximal humerus of Macropus giganteus (based on AMNH 35747): a. Showing markers for geometric morphometrics. (1) Posterolateral edge of greater tuberosity. (2) Posterior contact of greater tuberosity with head of humerus. (3) Most posterior point of curvature of the humeral head. (4) Posterior contact of lesser tuberosity and humeral head. (5) Posterior medial edge of lesser tuberosity. (6) Anteromedial edge of lesser tuberosity. (7) Anterior contact of lesser tuberosity and humeral head. (8) Anterior contact of greater tuberosity and humeral head. (9). Anterolateral edge of greater tuberosity. (10). Highest point along the ridge of the greater tuberosity. b. Showing measurements for the two-dimensional area analysis: Yellow = humeral head; Red = greater tuberosity; Blue = lesser tuberosity
In the CVA four locomotor groups (suspensory, arboreal, scansorial, and terrestrial) were assigned. A permutation test for the significance of the pairwise differences between groups was performed using Mahalanobis and Procrustes distances (Klingenberg and Monteiro 2005). The CVA was then repeated using IBM SPSS Statistics v15 including ricochetal taxa (extant macropodids plus Pedetes) and extinct macropodids as unknowns. This analysis provided a percentage of correct reclassifications using leaving-one-out cross-validation method and a probability of classification in one of the known locomotor categories for unknown taxa.
Two-Dimensional Area Measurements
The perimeters of the greater tuberosity, lesser tuberosity, and humeral head were defined with ImageJ, to measure the area of these structures in superior view (see Fig. 2b). The deep boundaries of the tuberosities were defined as a visible groove or change in bone texture where the tuberosity joins with the head of the humerus. The humeral head was defined as the articular surface of the scapulohumeral joint. Once measured, these values were summed to determine the total measured area; the areas of the greater tuberosity, lesser tuberosity, and humeral head were then normalized to the total measured area for each species, yielding dimensionless values for relative greater tuberosity area (rGTA), relative lesser tuberosity area (rLTA), and relative humeral head area (rHHA). These values were the focus of all subsequent analysis. The specimens were scored as belonging to one of the five locomotor modes described above: the sthenurine species were assigned to the category “unknown.”
A composite phylogenetic tree for all specimens studied (Fig. 3) was constructed based on published phylogenetic studies (Delsuc et al. 2002; Prideaux 2004; Koepfli et al. 2007; Blanga-Kanfi et al. 2009; Perelman et al. 2011; Nyakatura and Bininda-Emonds 2012; Llamas et al. 2015; May-Collado et al. 2015). First and last occurrence dates for all taxa were taken from the Paleobiology Database, to allow time scaling of the branches of the composite tree. Time scaling was performed using a custom R function (originally written and implemented by Napoli et al. [2017]), based on the phylogeny, first and last appearance dates, and estimates of birth, death, and sampling rates originally calculated for Mesozoic dinosaurs (Starrfelt and Liow 2016). If the first appearance date was not available, it was assumed to be the end of the Pleistocene epoch (11,700 years before present). The custom R function incorporated the ‘cal3timePaleoPhy’ function from the R package paleotree (Bapst 2012).
Phylogenetic tree of the taxa included in these analyses (see the Materials and Methods section for the references used to create this phylogeny). Key to colors: Red = extinct kangaroos. Orange = ricochetal taxa. Green = (regular) arboreal taxa. Purple = suspensory taxa. Blue = scansorial taxa. Black = terrestrial taxa
All following statistical analyses were performed in R, using RStudio (RStudio Team 2015). The ‘phylosignal’ function included with the R package picante (Kembel et al. 2010) was used to calculate a K statistic of phylogenetic signal for rGTA, rLTA, rHHA, and body mass. Phylogenetically independent contrasts of rGTA, rLTA, rHHA, and body mass were calculated using the ‘pic’ function of the R package ‘APE’ (Paradis et al. 2004). These contrasts were then subjected to standardized major axis linear regression via the ‘lmodel2’ function from the R package lmodel2 (Legendre 2018). Phylogenetic ANOVA (Garland et al. 1993) was conducted using the ‘aov.phylo’ function from the R package geiger (Pennell et al. 2014) to detect differences in rGTA, rLTA, and rHHA among the different locomotor categories, and to determine with which locomotor group the extinct kangaroos clustered.
Results
Geometric Morphometrics: Principal Components Analysis
The result obtained from the allometric test within a phylogenetic context was non-significant (p value of 0.188), therefore, there are not any allometric effects in our sample. The test for phylogenetic signal was significant (the K statistic is 0.236, p value is 0.001).
The first three components of the PCA together explained 56.3% of the variance (23.51%, 18.86%, and 13.91%, respectively). Figure 4 shows a plot of PC1 against PC2 (a plot of PC1 against PC3 did not appear significantly different with respect to sorting of taxa by locomotor mode). Figure S1 shows this same PCA with convex hulls drawn around the main locomotor categories: this highlights where the macropodids, extant and extinct, plot with respect to the other extant mammals sampled. The first component largely distinguishes more terrestrial taxa (with positive scores) from more arboreal ones (with negative scores), with the suspensory arboreal taxa in general having the most negative scores.
Principal Components Analysis performed from the 2D Procrustes coordinates. The symbols indicate different taxa, and the different colors indicate locomotor mode. The change in the shape of the proximal humerus is indicated along each axis: the lighter line shows the average shape. Numbers identifying certain taxa are shown in Table 1
The arboreal taxa tend to occupy the negative part of the morphospace along the first axis, and almost all have scores of less than +0.05. They are distinguished by a smaller greater tuberosity than the mean value, with especially with respect to the lesser (but more medial) projection of the cranial portion; a lesser tuberosity of similar size to the mean value, but oriented slightly more anteriorly; a deeper, more well-defined bicipital groove; and a humeral head that is more round than ovoid in shape with the apex projecting slightly laterally. The suspensory arboreal taxa mostly occupy the most negative portion of the morphospace along PC1, with scores mainly exceeding −1.0. Three suspensory taxa have less negative scores, clustering with the regular arboreal taxa: these include the two sloths (#s113,114), and the howler monkey, Alouatta seniculus (#85).
The terrestrial taxa tend to occupy the positive portion of the morphospace along the first axis, mostly with scores greater than 0.00 (taxa with more negative scores are discussed below). Terrestrial mammals are distinguished by a larger greater tuberosity than the mean value, especially with regard to the anterior (rather than medial) projection of the cranial portion; a lesser tuberosity slightly larger than the mean value, but oriented slightly more posteriorly; a more shallow, less well-defined bicipital groove; and a humeral head that is more ovoid than round in shape with the apex projecting slightly medially. The scansorial taxa fall in the middle of the morphospace on the first axis, and overlap with both terrestrial and arboreal taxa, although they tend to cluster more with the terrestrial taxa; almost all have scores of greater than ~ − 0.05.
The second principal component is not as easy to interpret, either in terms of the distribution of taxa or in terms of humeral shapes. The taxa with positive scores have a humerus with a relatively large lesser tuberosity, with a postero-medial projection of the caudal portion, and a relatively shallow bicipital groove; the apex of the humeral head projects slightly laterally. The greater tuberosity is of a similar size to the mean value but the most lateral portion of the caudal portion and the highest point along the crest are shifted slightly postero-laterally. The taxa with negative scores have almost precisely the opposite features. Although terrestrial taxa are spread throughout the second component, they are the only taxa with scores of less than −0.1 on this axis. In general, but by no means exclusively, the taxa with negative scores tend to be the carnivorans, and the taxa with positive scores tend to be the rodents, the xenarthrans, the non-suspensory primates, and the extant marsupials. This component may thus reflect phylogeny rather than locomotor function.
As previously mentioned, PC1 does not entirely distinguish arboreal from terrestrial and scansorial taxa, and these discrepancies appear to be related, at least in part, to phylogeny. Although no arboreal taxa have scores of greater than ~0.05 on PC1 (with a single exception, the Asiatic linsang Prionodon linsang [#72]), a number of them do plot in the positive portion of the morphospace along this axis. These include several carnivorans (the only arboreal felids Neofelis nebulosa [#65] and Leopardus wiedii [#59]; the fossa, Cryptoprocta ferox [#50]; one of the arboreal anteaters (Tamandua tetradactyla [#117]); and the porcupine (Erethizion dorsatum [#106]); and among the marsupials the cuscus (Phalanger sericius, [#22]), one of the largest of the possums, the tree-kangaroo (Dendrolagus goodfellowi [#9]), and the koala (Phascolarctos cinereus [#24]).
Terrestrial and scansorial taxa plotting in the arboreal portion of the morphospace, with negative scores exceeding −0.02, include the chinchillid caviomorph rodents (Chinchilla laniger [#100] and Lagidium peruanum [#101]); the Malagasy giant jumping rat (Hypogeomys antimena [#107]); many of the bears (including Ailuropoda melanoluca [#44], Melursus ursinus [#46], Ursus americanus [#48], and Ursus arctos [#49], although perhaps strangely the most arboreal of the bears, the spectacled bear Tremarctos ornatus [#47], clusters with the terrestrial taxa); some terrestrial musteloid carnivorans (Mustela nigripes [#38] and Spilogale putorius [#36]), and the armadillo (Dasypus kappleri [#111]), also cluster with the more arboreal taxa.
Extant and extinct kangaroos have divergent scores along the first PC axis. The extant taxa (plus Protemnodon spp.) plot almost entirely in the positive portion of the morphospace, mainly clustering with the scansorial taxa. The single exception is the gray kangaroo, Macropus giganteus [#11]), while the other large species of Macropus have slightly positive scores. Most of the extant kangaroos have similar scores of around zero along the second PC axis. The exceptions, with positive scores, are the quokka (Setonix brachyurus [#16]), the tree kangaroo (Dendrolagus goodfellowi [#9], here classified as arboreal), and the rufous bettong (Aepyprymnus rufescens [19]). The reasons for the departures of these taxa from the “kangaroo norm” are not clear. Note that although tree kangaroos appears similar to the other kangaroos in this two-dimensional planar view, they differ from them in having a humeral head that projects above the level of the tuberosities (Warburton et al. 2011), a general feature of arboreal mammals (Argot 2001).
In contrast, the sthenurines plot entirely in the negative portion of the morphospace along PC1, clustering with the suspensory taxa, and have divergent scores along PC2. Sthenurines also resemble suspensory primates (Arias-Martorell 2018), as well as tree kangaroos (see above), in having humeral heads that project above the level of the tuberosities (Wells and Tedford 1995). The sole non-macropodoid ricochetal taxon (see Cavagna and Legramandi 2015) is the springhare (Pedetes capensis [#96]), a large (~3 kg) rodent, which clusters closer to the sthenurines than to the macropodoids. (Note that our specimen of Pedetes is the same individual as in the tables of Cavagna and Legramandi 2015!)
Geometric Morphometrics: Canonical Variates Analysis
A preliminary canonical variates analysis (not shown) using only three locomotor categories (arboreal, scansorial, and terrestrial) and including only carnivorans (excluding ursids), rodents and primates, showed that proximal humeral morphology could distinguish locomotor mode to a certain extent. Arboreal forms could be distinguished from the terrestrial plus scansorial taxa, and 81.9% (57.1% with cross-validation) of the cases were correctly reclassified.
Figure 5 shows a CVA of the entire dataset, with all macropodids (plus Pedetes capensis) entered as unknowns; Table 2 shows the % classification of the “unknowns” into the different locomotor categories; Fig. S2 shows this same CVA with convex hulls around the main locomotor categories. The inclusion of the suspensory forms, which can be distinguished as a discrete group in the positive portion of the first axis, results in slightly different separation of the other locomotor groups from our preliminary analysis: now the terrestrial forms are distinct, with scores in the negative portion of the first axis, while the scansorial and (non-suspensory) arboreal forms are not distinguishable from each other. This observation is confirmed by the statistical results of the permutation tests for Mahalanobis distances among groups: suspensory and terrestrial forms are statistically different from each other and the other groups (all p values are below 0.0001), but scansorial and non-suspensory arboreal forms are only slightly different (p value 0.0276) (see Table 2). The second axis does not appear to have any locomotor or phylogenetic component, apart from the fact that most of the sthenurines have high positive scores. The percent reclassification is poorer than in the preliminary analysis, with only 54.7% correct reclassification of cases using leaving-one-out cross-validation method.
Principal Components Analysis performed from the 2D Procrustes coordinates. The symbols indicate different taxa, and the different colors indicate locomotor mode. The change in the shape of the proximal humerus along the first axis is similar to, but in the opposite direction from, the change the change along first component of the PCA
The suspensory taxa all have positive scores, and in this analysis the sloths cluster with the primates. In most cases suspensory forms have higher positive scores than all of the other taxa (although note the high positive score of the uakari [Cacajao calvus, #88]), but the howler monkey (Alouatta seniculus, [#85]), possibly the most terrestrial of the primates classified here as suspensory, has scores within the range of several non-suspensory mammals, as in the PCA. There are a few anomalous placements of other taxa in the morphospace. As in the PCA, some terrestrial rodents cluster with more arboreal forms in the positive portion of the first axis: the chinchilla (Chinchilla laniger [#100]), the viscachia (Lagidium peruanum [#101]), and the Malagasy giant jumping rat (Hypogeomys antimena [#107]). Similarly, some terrestrial carnivorans cluster with more arboreal forms: these include the black bear (Ursus americanus [#49]) plus the black-footed ferret (Mustela nigripes [#38]), which cluster with the arboreal forms in the PCA; but now also a couple of other taxa, the spotted skunk (Spilogale putorius [#36]), and the panda (Ailuropoda melanoleuca [#44]) also cluster in this portion of the morphospace. One scansorial carnivoran, the grey fox (Urocyon cinereoargentus [#34], the only non-terrestrial canid) clusters with the terrestrial taxa.
When the macropodids (plus the ricochetal rodent Pedetes) are entered as unknowns, a clear separation is seen between the sthenurines (clustering with the suspensory taxa) and the other macropodids (including the extinct Protemnodon spp.), which cluster with the arboreal and scansorial taxa. The placement of the sthenurines echoes that seen in the PCA: the smallest sthenurine (“Procoptodon” gilli) has the least positive of the scores on the first axis (although it has a higher positive score on the second axis than any extant macropodid), in a similar position to the springhare (Pedetes capensis [#96]), and one of the large sthenurines (Sthenurus stirlingi [#6]) has a negative score on the second axis (all of the other sthenurines have positive scores). Table 3 shows that while the probability of most of the sthenurines clustering with the suspensory taxa is high (~90% or greater), the errant S. stirling has only a 62% probability, and “Procoptodon” gilli only 21%, while the probability for Pedetes is 35%.
The extant macropodids cluster in the middle of the morphospace, as in the PCA, with weakly negative scores to very weakly positive scores on the first axis, and negative scores on the second axis. In contrast to the sthenurines, macropodines are mostly reclassified as arboreal or scansorial forms, and the probability of being a suspensory form is usually <1% (the only exception being the quokka, Setonix brachyurus [#16], with a 6% probability). (As in the PCA, although not to such an extreme extent, the rufous rat-kangaroo, Aepyprymnus rufescens [#19], is the anomalous taxon on the second axis with a positive score.) The only kangaroos with more positive scores on the first axis (> 1) are the quokka and the burrowing bettong (Bettongia lesueur [#20]). The two species of Protemnodon (#s7,8) group away from the sthenurines and plot close to some of the extant macropodids.
Two-Dimensional Area Measurements
The relative values of the areas of the components of the proximal humeral head in taxa of the different locomotor categories are shown in Fig. 6, and Table 4 summarizes the results of statistical analyses discriminating among locomotor modes based on the three component head areas and the K statistics phylogenetic signal for those components.
Boxplots showing the range of values of relative areas proximal humeral component areas for the different locomotor categories. The midline = the median, box margins indicate the 25th and 75th percentiles, the whiskers extend to the 10th and 90th percentiles. a. Relative greater tuberosity area (rGTA). b. Relative lesser tuberosity area (rLTA). c. Relative humeral head area (rHHA)
The rGTA was found to differ significantly between arboreal/suspensory taxa and terrestrial taxa (p < 0.05). Arboreal and suspensory taxa generally showed lower rGTA values than ricochetal, scansorial, and terrestrial taxa. Sthenurine kangaroos tended to have rGTA values most similar to arboreal/suspensory taxa, and were fairly distinct from the range of terrestrial, scansorial, and ricochetal taxa; however, this difference was not statistically significant, likely as a result of the small number of sthenurine specimens sampled. Protemnodon spp. were not included in this analysis due to the sample size of two, although Protemnodon anak is figured in the Ternary plot (Fig. 7) to illustrate its position relative to other macropodoids. The significance tests were performed using a phylogenetic ANOVA, as implemented in the R package geiger; this method simulates trait evolution along a given phylogenetic tree to empirically derive a null distribution to which the F statistic of the dataset is compared to assess significance, incorporating phylogenetic nonindependence into the hypothesis test.
Ternary plots of relative areas of proximal humeral components. Key to colors indicating locomotor mode as for Figs. 3, 4, and 5. The single ricochetal taxon clustering with the sthenurines and suspensory taxa is the springhare, Pedetes capensis. The red diamond clustering with the other richochetal taxa is Protemnodon anak
The rLTA did not differ significantly between arboreal, suspensory, scansorial, terrestrial, or sthenurine taxa. Ricochetal taxa were found to have relatively larger lesser tuberosities than all other groups (p < 0.05: Fig. 6). Sthenurines had the smallest lesser tuberosities of any grouping, with the next smallest mean rLTA belonging to suspensory taxa; however, the difference between sthenurines and the other groups (except for the ricochetals) was generally not statistically significant, except between sthenurines and arboreal climbers.
The rHHA values were significantly greater in sthenurines than in both ricochetal and terrestrial taxa (p < 0.05; Fig. 6). While arboreal and suspensory taxa did not differ significantly from other locomotor groups, they tended to have high rHHA values; this was most evident in suspensory taxa.
The K statistic of phylogenetic signal for the relative area of the greater tuberosity (rGTA) was 0.272 (p < 0.05). The K statistic for the relative area of the lesser tuberosity (rLTA) was 0.330 (p < 0.05), and for the relative area of the greater tuberosity (rHHA) was 0.244 (p < 0.05). All of these K statistics were statistically significant and below 1, indicating that closely related species in our dataset show greater differences in humeral morphology than would be expected by chance (phylogenetic overdispersion). Phylogenetic overdispersion occurs when selection causes traits to differ more between related taxa than expected under a random-walk through trait space, and here supports our inference that humeral head areas correlate to locomotor modes. Body mass showed a similar pattern, with a K statistic of 0.451 (p < 0.05). Standardized major axis regressions of phylogenetically independent contrasts of rGTA, rLTA, and rHHA against body mass found uniformly insignificant relationships (p > 0.05), with R2 values less than 0.1.
Visualization of rGTA, rLTA, and rHHA in ternary space (Fig. 7) is helpful to see which values tend to discriminate between locomotor groups. As discussed above, rGTA generally separates arboreal and suspensory taxa from scansorial and terrestrial taxa, with ricochetals occupying intermediate space. The major discriminant between sthenurines and ricochetals is rLTA, with the ricochetal taxa possessing far larger rLTA values. High rHHA values tend to be found in arboreal and suspensory taxa and sthenurines, with ricochetals, scansorials, and terrestrials having lower values.
Although the sthenurines analyzed here are all fairly large (and sthenurines generally tend to be more massive than extant macropodines), our findings are not the result of allometry. The relative areas rGTA, rLTA, and rHHA did not scale significantly with body mass, and extant macropodines all show great similarity irrespective of size. The characteristic humeral head morphology of sthenurines appears to be specific to their lineage, resembling arboreal suspensory climbers more than any other kangaroo in our dataset.
Discussion
Our results on extant mammals show that proximal humeral morphology changes in a characteristic fashion along a locomotor spectrum from animals that engage in less weight-bearing on their forelimbs to those that continuously bear weight on their forelimbs (i.e., from suspensory, through arboreal and scansorial, to terrestrial taxa). The results obtained here indicate that sthenurine kangaroos possessed a proximal humeral morphology distinct from that of extant macropodids, which we interpret as indicating a difference in the mode of locomotion.
In general, the more terrestrial the taxon, the larger the size of the tuberosities (especially the greater tuberosity), and the more ovoid the shape of the humeral head. This is functionally related to the need, in more terrestrial taxa, to stabilize the glenohumeral joint during weight-bearing by means of larger rotator cuff muscles, and the restriction of the motion of the forelimb to the parasagittal plane. In contrast, smaller tuberosities and a rounder humeral head allow for greater forelimb mobility, important in more arboreal taxa for reaching and grasping. The morphology of the proximal humerus in any given taxon reflects a compromise between support and agility, a compromise that will have different demands depending on the type of locomotion.
In both the PCA (Fig. 4) and the CVA (Fig. 5), the scores from 2D geometric morphometric analysis show that suspensory taxa are have a morphology that is distinctly different from other locomotor types. Although there is considerable overlap among the other locomotor types (especially between arboreal and scansorial), the general distribution pattern along the first axis is the same in both analyses. In the PCA, the distribution of taxa from negative to positive is suspensory, arboreal, scansorial, terrestrial, and the CVA shows the same distribution pattern (but from positive to negative).
Of particular interest is the way in which the macropodids separate along the first axis in both analyses. The extant macropodids (plus the extinct protemnodons) cluster in the middle of the morphospace in the region of the overlap of arboreal and scansorial taxa. In contrast, the sthenurines cluster at the negative (PCA) or positive (CVA) end of the morphospace in the region occupied by the suspensory taxa. The resemblance of the sthenurine proximal humeral morphology to that of suspensory primates can also be seen in Figs. 1b and 8b. Interestingly, the one sthenurine that overlaps in scores with non-suspensory taxa in both geometric morphometric analyses is the relatively small species (~55 kg, Helgen et al. 2006) “Procoptodon” gilli (#2), which also tended to cluster with extant kangaroos in analyses of the hind limb bones (Janis et al. 2014), and its calcaneal morpohology shows greater similarities to those of large macropodids than to other sthenurines (Bishop 1997). In contrast, the similarly-sized “Procoptodon” browneorum here clusters with the other sthenurines. It is not clear why one of the specimens of Sthenurus stirlingi (#6) differs from the other sthenurines in having negative scores on the second axis in both the PCA and the CVA: it has a relatively modest greater tuberosity, but it does have a very small and posteriorly-projecting lesser tuberosity.
Unlike the other rodents, the ricochetal Pedetes capensis (the springhare [#96]), has a proximal humeral morphology similar to that of the sthenurine kangaroos, and plots near to them in the PCA morphospace. Springhares use their forelimbs for excavating burrows, and may support their weight on their forelimbs while moving slowly during foraging (Butynski and Kalina 2013). Anthropologist Robin Crompton, who has observed springhares in the field, noted (personal communication to CMJ) that, unlike small macropodids (Windsor and Dagg 1971), springhares do not appear to have a dedicated quadrupedal bounding gait.
The species of Protemnodon (#s7,8) plot in the same general region of the PCA morphospace as the extant kangaroos along the first axis, but differ in having much more negative scores on PC2. This placement is likely because they possess a large, hook-like anterior projection of the cranial portion of the greater tuberosity (see Fig. 8c), which they share with a number of terrestrial carnivorans with scores in this area of the morphospace (e.g., the Canadian lynx, Lynx canadensis [#61] and the small Indian civet, Viverricula indica [#79]; see also the fanaloka [Fossa fossana] in Fig. 1d). Note that the thylacine, Thylacinus cynocephalus (#28), also has similar scores on PC2 to Protemnodon spp.), although it has slightly more positive scores on PC1. However, in the CVA the Protemnodon species plot with some of the extant macropodines, and are separated along both axes from the terrestrial carnivorans and the thylacine.
With respect to the areas of the different components of the proximal humerus, sthenurines differ from almost all mammals in having relatively smaller lesser tuberosities (see Figs. 6 and 8b) and relatively larger humeral heads (although, perhaps strangely, in the PCA analysis it is the orientation rather than the size of the lesser tuberosity that distinguishes these taxa with negative scores on the first axis). The results of the ternary plots of humeral areas (Fig. 7) are similar to those obtained with the geometric morphometric analyses: sthenurines cluster with arboreal and especially suspensory taxa, while other macropodids (including Protemnodon) plot in the area of overlap between arboreal, scansorial, and terrestrial taxa.
In the component area analysis, extant macropodids display consistently large (and medially directed) lesser tuberosities, indicative of a large subscapularis muscle, important as a medial rotator of the humerus. As previously discussed, an enlarged and medially-directed lesser tuberosity is seen in some arboreal mammals, where it may be related to the use of the subscapularis in vertical climbing (Sargis 2002). However, a large subscapularis is also important in knuckle-walking hominoids, with medial rotation of the humerus effected during the support phase (Arias-Martorell 2018). Issues of shoulder stabilization during quadrupedal locomotion are different for hominoids than other mammals, due to their scapula being placed dorsally on an expanded ribcage, resulting in the scapular glenoid facing laterally rather than ventrally, with the resulting tendency of the humerus to be displaced dorsally (Bello-Hellegouarch et al. 2012). An enlarged subscapularis in macropodids may be related to their use of the forelimbs in pentapedal locomotion. In contrast, the ricochetal rodent, Pedetes, does not have an enlarged lesser tuberosity; in all of the analysis it plots near to the sthenurines.
All of our analyses show that sthenurine kangaroos have a very different morphology of the proximal humerus from that seen in other macropodids, extant and extinct: rather, their morphology resembles that of extant mammals that engage in suspensory climbing. What can we conclude from this about sthenurine locomotion? Among extant taxa, does this humeral morphology reflect the use of the arm (especially mobility) in under-branch hanging, a lesser degree of weight-bearing on the forearms, or both? Suspensory mammals may engage in quadrupedal terrestrial gaits on occasion, in particular (among the taxa in our dataset) chimpanzees, howler monkeys, and woolly monkeys (Fleagle 2013). But in general, suspensory mammals would be expected to spend less locomotor time in forelimb weight-bearing than other taxa. (In contrast, terrestrial taxa spend all of their locomotor time in forelimb weight-bearing, and they are consistently placed at the opposite end of the morphospace to the suspensory taxa in the geometric morphometric analyses.) Humans are the only primates that, at least as adults, do not engage in forelimb weight-bearing locomotion. We did not include Homo sapiens in our sample, but we note that the human proximal humerus differs little in morphology from that of other hominoids, although it does show a somewhat lesser degree of torsion (Aiello and Dean 1990).
Might the morphology of the sthenurine humerus merely reflect the degree of forelimb mobility practiced by suspensory taxa during overhead grasping in locomotion in the tree canopies? Based on a number of lines of anatomical evidence, sthenurines are hypothesized to have engaged in overhead browsing behavior, in which they reached upward their arms to feed on leaves (Wells and Tedford 1995). The functional demands on the humeral head by sthenurines engaged in overhead browsing are likely similar to those experienced during suspensory locomotion; both behaviors involve extensive rotation of the humerus to allow for maximal reach, although of course sthenurines would not have been suspending their body weight on their forelimbs. Therefore, the similarities in proximal humeral morphology between sthenurines and arboreal and suspensory taxa support the overhead browsing model of their ecology.
We argue here that sthenurine humeral morphology additionally supports the hypothesis that they did not habitually support their body weight on the forelimbs in the type of quadrupedal or pentapedal slow walking gait seen in extant kangaroos. Various other features of sthenurine anatomy have led the proposal that they did not have a forelimb-supported walking gait (see Wells and Tedford 1995, who comment on [p. 83] “the emancipation of the forelimb from a locomotory role”). Our observations on proximal humeral morphology can be added to their observations, which include: a reduced olecranon process that would restrict the ability of the triceps muscle to resist elbow flexion in supporting body weight or in providing locomotor propulsion; a relatively smaller cross-sectional area of the humerus; and a less well-developed pectoral crest. The highly stiffened nature of the sthenurine lumbar region would also limit the ability for dorsiflexion in placing the hands on the ground (Wells and Tedford 1995). Note, however, that pentapedal (versus quadrupedal) locomotion is limited to a single clade of derived macropodines (the genera Macropus, Wallabia, and Onychogalea) (Dawson et al. 2015), so any forelimb-supported slow gait in sthenurines would not be expected to necessarily employ tail support. Indeed, Dawson (2015) has shown that the morphology of the sthenurine anterior caudal vertebrae would preclude the drawing of the tail under the body that is essential for tail-supported pentapedal locomotion in macropodines.
We consider that another important difference between sthenurines and other macropodids, again relating to their probable locomotor habits, is the relative size of the supraspinatus muscle. The scapula of sthenurines is unusual in having a highly reduced supraspinous fossa, the area of origin of the supraspinatus muscle (Wells and Tedford 1995; see also Sears 2005). Wells and Tedford (1995:84) described the sthenurine scapula as “curiously human-like,” and proposed that this morphology enabled sthenurines to raise their forelimb above their head. (We note, however, that although sthenurine forelimbs were undoubtedly highly mobile, they lack a key anatomical feature that may allow for this behavior in extant hominoid primates [and suspensory New World monkeys]: that is, a scapula positioned dorsally on an expanded ribcage, with a laterally-facing glenoid [Aiello and Dean 1990].) Note also that, while sthenurines resemble humans in their extremely reduced supraspinous fossa, this morphology is not typical of other anthropoids, which retain a large supraspinous fossa and in whom the supraspinous muscle is important for shoulder stabilization during terrestrial quadrupedal locomotion (Bello-Hellegouarch et al. 2012). Thus the “human-like” nature of the sthenurine scapula might indicate a glenohumeral joint that was not adapted to weight-bearing, as is the case in humans.
Protemnodon spp. do not plot as being distinctly different in humeral morphology from other macropodids in the geometric morphometric analyses, although they do possess a carnivoran-like morphology of the greater tuberosity, with a cranial tip that is expanded antero-medially (Fig. 8c). This might be indicative of an enlarged area of insertion for the supraspinatus, although the supraspinous fossa on the scapula is of a similar size to that of extant macropodids (see Den Boer 2018). The position of Protemnodon anak in the ternary plot (Fig. 7) also indicates a relatively larger greater tuberosity, but a smaller lesser tuberosity, than most extant macropodoids. Thus Protemnodon evinces aspects of the proximal humerus that may be indicative of more extensive weight-bearing on the forelimbs than in extant macropodids, perhaps suggestive of a greater degree of quadrupedal activity, as suggested by Den Boer (2018).
Conclusions
Extant macropodoids use quadrupedal or pentapedal locomotion at slow speeds, during which weight is borne on their forelimbs, but (with the exception of the musky rat-kangaroo, Hypsiprymnodon moschatus) at faster speeds they switch to bipedal hopping, when their weight is borne entirely on their hind limbs. Their humeral morphology resembles that of scansorial (semi-terrestrial) taxa, reflecting the fact that they do not bear weight on their forelimbs during fast locomotion. They are unique in possessing a relatively large lesser tuberosity, which may reflect the use of the subscapularis muscle during quadrupedal/pentapedal walking.
The extinct kangaroos studied here have a proximal humerus morphology that differs from that of the extant ones, indicative of different modes of locomotion relating to the extent of weight-bearing on the forelimbs. The sthenurines (“Procoptodon,” Simosthenurus, and Sthenurus) have a humeral morphology resembling that of suspensory arboreal taxa, supporting the hypothesis based on other anatomical features that they lacked the dedicated quadrupedal or pentapedal walking gait of extant macropodids, where weight is borne on the forelimbs (Wells and Tedford 1995), and may instead have engaged in bipedal striding at slow speeds (Janis et al. 2014). We note that preliminary trackway data from the Pliocene of central Australia (Camens and Worthy 2019) also supports this hypothesis of bipedal striding in sthenurines. Protemnodon has a proximal humeral morphology resembling that of scansorial or terrestrial carnivorans, supporting the hypothesis of a predominantly quadrupedal or pentapedal mode of locomotion at all speeds (Kear et al. 2008; Den Boer 2018).
Summary
The morphology of the proximal humerus can be shown to differ between different modes of locomotion in therian mammals – arboreal, suspensory, scansorial, and terrestrial – and suspensory forms are clearly distinguishable from all others. The differences in morphology reflect the functional requirements of the glenohumeral joint in locomotion. The more arboreal taxa (bearing their body weight on their forelimbs less frequently) have rounded humeral heads and smaller tuberosities for muscle insertion, reflecting the demands of mobility of the shoulder joint over stability. The more terrestrial taxa (bearing their body weight on their forelimbs in all locomotor activities) have more ovoid humeral heads and larger tuberosities (especially the greater tuberosity), reflecting the demands of stability of the shoulder joint over mobility.
Extant macropodoids (kangaroos and rat-kangaroos), which bear weight on their forelimbs only during their slow quadrupedal or pentapedal gaits, have a proximal humeral morphology that mostly resembles that of the scansorial (semi-terrestrial) taxa. However, the extinct macropodids do not plot with the extant ones. The sthenurines plot with the suspensory taxa, evincing a humeral morphology reflecting a shoulder joint favoring mobility over stability. The Protemnodon species plot in a similar area of the morphospace as the extant macropodoids along the first axis of the Principal Components Analysis, but do not group with them along the second axis, instead grouping with scansorial and terrestrial carnivorans that possess a large greater tuberosity. These results support prior hypotheses of locomotion in these extinct kangaroos: bipedal striding locomotion (especially during slow locomotion) in sthenurines and predominantly quadrupedal/pentapedal locomotion in Protemnodon.
References
Adams DC, Otárola-Castillo E (2013) Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399. doi:https://doi.org/10.1111/2041-210X.12035
Aiello L, Dean C (1990) An Introduction to Human Evolutionary Anatomy. Academic Press, New York
Argot C (2001) Functional-adaptive anatomy of the forelimb in the Didelphidae, and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol 247:51–79
Arias-Martorell J (2018) The morphology and evolutionary history of the glenohumeral joint of hominoids: a review. Ecol Evol 2018:1–20. doi:https://doi.org/10.1002/ece3.4392
Bapst DW (2012) Paleotree: an R package for paleontological and phylogenetic analyses of evolution. Methods Ecol Evol 3:803–807. doi: https://doi.org/10.1111/j.2041-210X.2012.00223.x.
Baudinette RV (1989) The biomechanics and energetics of locomotion in Macropodoidea. In: Grigg G, Jarman P, Hume I (eds) Kangaroos, Wallabies, and Rat-Kangaroos. Surrey Beatty & Sons Pty Limited, New South Wales, pp 245–253
Bello-Hellegouarch G, Potau JM, Arias-Martorell J, Pastor JF, Diogo R, Pérez- Pérez A (2012) The rotator cuff muscles in Hominoidea: evolution and adaptations to different types of locomotion. In: Hughes EF, Hill ME (eds) Primates: Classification, Evolution and Behavior. Nova Science Publishers, Hauppauge, pp 111–134
Bennett MB (2000) Unifying principles in terrestrial locomotion: do hopping Australian mammals fit in? Physiol Biochem Zool 73:726–735
Bennett MB, Taylor GC (1995) Scaling of elastic strain energy in kangaroos and the benefits of being big. Nature 378:56–69
Blanga-Kanfi S, Miranda H, Penn O, Pupko C, DeBry RW, Huchon D (2009) Rodent phylogeny revised: analysis of six nuclear genes from all major rodent clades. BMC Evol Biol 9:71. doi: https://doi.org/10.1186/1471-2148-9-71
Bishop N (1997) Functional anatomy of the macropodid pes. Proc Linn Soc N S W 117:17–50
Burk A, Westerman M, Springer M (1998) The phylogenetic position of the musky rat-kangaroo and the evolution of bipedal hopping in kangaroos (Macropodidae: Diprotodontia). Syst Biol 47:457–474
Butynski TM, Kalina J (2013) Family Pedetidae: springhares. In: Kingdon J, Happold DCD (eds) Mammals of Africa Volume III: Rodents, Hares and Rabbits. Bloomsbury, London, pp 618–627
Camens AB, Worthy, TH (2019) Walk like a kangaroo: new fossil trackways reveal a bipedally striding macropodid in the Pliocene of Central Australia. J Vertebr Paleontol, Programs and Abstracts 2019:72
Cavagna GA, Legramandi MA (2015) Running, hopping and trotting: tuning step frequency to the resonant frequency of the bouncing system favors larger animals. J Exp Biol 218:3276–3283. doi:https://doi.org/10.1242/jeb.127142
Dawson RS (2015) Morphological correlates of pentapedal locomotion in kangaroos and wallabies (Family: Macropodidae). Dissertation, The University of Western Australia, Perth
Dawson RS, Warburton NM, Richards HL, Milne N (2015) Walking on five legs: investigating tail use during low gait in kangaroos and wallabies. Aust J Zool 63:192-200. doi:https://doi.org/10.11071/ZO15007
Dawson TJ (1995) Kangaroos: Biology of the Largest Marsupials. Comstock Publishing Associates, New York
Delsuc F, Scally M, Madsen O, Stanhope MJ, de Jong WW, Catzeflis FM, Springer MS, Douzery EJP (2002) Molecular phylogeny of living xenarthrans and the impact of character and taxon sampling on the placental tree rooting. Mol Biol Evol 19:1656–1671. doi: https://doi.org/10.1093/oxfordjournals.molbev.a003989.
Den Boer W (2018) Evolutionary progression of the iconic Australasian kangaroos, rat-kangaroos, and their fossil relatives (Marsupialia: Macropodiformes). Dissertation, Uppsala University, Uppsala
Doube M, Felder AA, Chua MY, Lodhia K, Klosowski MM, Hutchinson JR, Shefelbine SJ (2018) Limb bone scaling in hopping macropods and quadrupedal artiodatyls. Roy Soc Open Sci 5:180152. doi: https://doi.org/10.1098/rsos.180152
Dryden IL, Mardia K (1998) Statistical Analysis of Shape. John Wiley and Sons, Chichester
Fleagle JG (2013) Primate Adaptation and Evolution, third edition. Academic Press, San Diego
Garland TJ Jr, Dickerman AW, Janis CM, Jones JA (1993) Phylogenetic analysis of covariance by computer simulation. Syst Biol 42:265–292
Gebo DL, Rose KD (1993) Skeletal morphology and locomotor adaptation in Prolimnocyon atavus, an early Eocene hyaenodonid creodont. J Vertebr Paleontol 13:125–144
Gebo DL, Sargis EJ (1994) Terrestrial adaptations in the postcranial skeletons of guenons. Am J Phys Anthropol 59:175–193
Helgen K, Wells R, Kear B, Gerdtz W, Flannery T (2006) Ecological and evolutionary significance of sizes of giant extinct kangaroos. Aust J Zool 54: 293–303. doi:https://doi.org/10.1071/ZO05077
Janis CM, Buttrill K, Figueirido B (2014) Locomotion in extinct giant kangaroos: were sthenurines hop-less monsters? PLoS ONE 9(10): e109888. doi:https://doi.org/10.1371/journal.pone.0109888
Jenkins FA Jr, Weijs WA (1979) The functional anatomy of the shoulder in the Virginia opossum (Didelphis virginiana). J Zool 188:379–410
Jones KE, Bielby J, Cardillo M, Fritz SA, O’Dell J, Orne CDL, Safi K, Sechrest W, Boakes EH, Carbone C, Cutts MJ, Foster JK, Greyner R, Habib M, Plaster CA, Price SA, Rigby EA, Rist J, Teacher A, Bininda-Emonds O, Gittleman J, Mace GN, Purvis A (2009) PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90:2648. doi:https://doi.org/10.1890/08-1494.1
Kear B (2002) Phylogenetic implications of macropodid (Marsupialia: Macropodoidea) postcranial remains from Miocene deposits of Riversleigh, northwestern Queensland. Alcheringa 26:299-318. doi:https://doi.org/10.1080/03115510208619259
Kear BP, Archer M, Flannery TF (2001) Bulungamayine (Marsupiala: Macropodoidea) postcranial elements from the late Miocene of Riversleigh northwestern Queensland. AAP Memoirs 25:103–122
Kear BP, Lee MSY, Gerdtz WR, Flannery TF (2008) Evolution of hind limb proportions in kangaroos (Marsupalia: Macropodoidea). In: Sargis EF, Dagosto M (eds) Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Springer, New York, pp 25–55
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464. doi:https://doi.org/10.1093/bioinformatics/btq166
Klingenberg CP (2011) MorphoJ. Faculty of Life Sciences, University of Manchester, Manchester. Available from: http://www.flywings.org.uk/MorphoJ_page.htm. Accessed 10 March 2016
Klingenberg CP, Monteiro LR (2005) Distances and directions in multidimensional shape spaces: implications for morphometric applications. Syst Biol 54:678–688. doi:https://doi.org/10.1080/10635150590947258
Koepfli K-P, Compper ME, Eizirik E, Ho C-C, Linden L, Madlonado JE, Wayne RK (2007) Phylogeny of the Procyonidae (Mammalia: Carnivora): molecules, morphology and the great American interchange. Mol Phylogenet Evol 43:1076–1095. doi: https://doi.org/10.1016/j.ympev.2006.10.003
Larson SG, Stern JT Jr (1989) The role of the supraspinatus in the quadrupedal locomotion of vervets (Cercopithecus aethiops): implications for the interpretation of humeral morphology. Am J Phys Anthropol 79:369–377
Legendre P (2018) lmodel2: Model II Regression. https://CRAN.R-project.org/package version 1.7.3. Accessed 3 Sept 2018
Llamas B, Brotherton P, Mitchell KJ, Templeton JEL, Thomson VA, Metcalf JL, Armstrong KN, Kasper M, Richards SM, Camens AB, Lee MSY, Cooper A (2015) Late Pleistocene Australian marsupial DNA clarifies the affinities of extinct megafaunal kangaroos and wallabies. Mol Biol Evol 32:574–584. doi:https://doi.org/10.1093/molbev/msu338
McGowan CP, Skinner J, Biewener AA (2008) Hind limb scaling of kangaroos and wallabies (superfamily Macropodoidea): implications for hopping performance, safety factor, and elastic scaling. J Anat 212:153–163. doi:https://doi.org/10.1111/j.1469-7580.2007.00841.x
Mathewson MA, Kwan A, Eng CA, Lieber RL, Ward SR (2014) Comparison of rotator cuff muscle architecture between humans and other selected vertebrates. J Exp Biol 217:261–273. doi:https://doi.org/10.1242/jeb.083923
May-Collado LJ, Kilpatrick CW, Agnarsson I (2015) Mammals from “down under”: a multi-gene species-level phylogeny of marsupial mammals (Mammalia, Metatheria). PeerJ 3: e805. doi: https://doi.org/10.7717/peerj.805.
Morgan CC, Álverez A (2013) The humerus of South American caviomorphs rodents: shape, function and size in a phylogenetic context. J Zool 290:107–116. doi:https://doi.org/10.1111/jzo.12017
Murray PF (1991) The sthenurine affinity of the late Miocene kangaroo, Hadronomas puckridgi Woodburne, 1967 (Marsupialia: Macropodidae). Alcheringa 15:255–283
Napoli JG, Williamson TE, Shelley SL, Brusatte SL (2017) A digital endocranial cast of the early Paleocene (Puercan) ‘archaic’ mammal Onychodectes tisonensis (Eutheria: Taeniodonta). J Mammal Evol 25(2):179–195. doi: https://doi.org/10.1007/s10914-017-9381-1
Nyakatura K, Bininda-Emonds OR (2012) Updating the evolutionary history of Carnivora (Mammalia): a new species-level supertree complete with divergence time estimates. BMC Biol 10:12. doi: https://doi.org/10.1186/1741-7007-10-12
O'Connor S, Dawson T, Kram R, Donelan J (2014) The kangaroo's tail propels and powers pentapedal locomotion. Biol Lett 10: 20140381. doi:https://doi.org/10.1098/rsbl.2014.0381
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. doi: https://doi.org/10.1093/bioinformatics/btg412
Pennell MW, Eastman JM, Slater GJ, Brown JW, Uyed JC, FitzJohn RG, Alfaro ME, Harmon LJ (2014) Geiger v2.0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics 15: 2216–2218
Perelman P, Johnson WE, Roos C, Seuánez HN, Horvarth JE, Moreira MAM, Kessing B, Pontius J, Roelke M, Rumpler Y, Schneider MPC, Silva A, O’Brien SJ, Pecon-Slattery J (2011) A molecular phylogeny of living primates. PLoS Genet 7(3): e1001342. doi: https://doi.org/10.1371/journal.pgen.1001342
Prideaux G (2004) Systematics and evolution of the sthenurine kangaroos. Univ Cal Publ Geol Sci 46:1–623
Rose MD (1989) New postcranial specimens of catarrhines from the middle Miocene Chinji Formation, Pakistan: descriptions and a discussion of proximal humeral functional morphology in anthropoids. J Hum Evol 18:131–162
Ride WDL, Pridmore PA, Barwick RE, Wells RT, Heady RD (1997) Towards a biology of Propleopus oscillans (Marsupialia: Propleopinae: Hypsiprymnodontidae). Proc Linn Soc N S W 117:243–328
Rohlf FJ (2016a) Tps-Util program, version 1.68. Department of Ecology & Evolution, State University of New York
Rohlf FJ (2016b) Tps-dig 2.25. Department of Ecology and Evolution, State University of New York
RStudio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston. Available at: http://www.rstudio.com/. Accessed 20 Jan 2016
Salton JA, Sargis EJ (2008) Evolutionary morphology of the Tenrecoidea (Mammalia) forelimb skeleton. In: Sargis EF, Dagosto M (eds) Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Springer, New York, pp 51–71
Sargis EJ (2002) Functional morphology of the forelimb of tupaiids (Mammalia, Scandentia) and its phylogenetic implications. J Morphol 253:10–42
Schmitt D (2003) Mediolateral reaction forces and forelimb anatomy in quadrupedal primates: implications for interpreting locomotor behavior in fossil primates. J Hum Evol 44:47–58. doi:https://doi.org/10.1016/S0047-2484(02)00165-3
Sears KE (2005) Role of development in the evolution of the scapula of the giant sthenurine kangaroos (Macropodidae: Sthenurinae). J Morphol 265:226–236. doi:https://doi.org/10.1002/jmor.10353
Snelling EP, Biewener AA, Hu Q, Taggart DA, Fuller A, Mitchell D, Maloney SK, Seymour RS (2017) Scaling of the ankle extensor muscle-tendon units and the biomechanical implications for bipedal hopping locomotion in the post-pouch kangaroo Macropus fuliginousus. J Anat 231:921–930. doi:https://doi.org/10.1111/joa.12715
Sonnabend DH, Young AA (2009) Comparative anatomy of the rotator cuff. J Bone Joint Surg 91-B:1632–1637. doi:https://doi.org/10.1302/0301-620X.91B12.22370
Starrfelt J, Liow LH (2016) How many dinosaur species were there? True richness estimated using a Poisson sampling model (TRiPS). Philos Trans R Soc B: 371, 20150219. doi:https://doi.org/10.1017/CBO9781107415324.004
Szalay FS, Dagosto M (1980) Locomotor adaptations as reflected on the humerus of Paleogene primates. Folia Primatol 34:1–45
Szalay FS, Sargis EJ (2001) Model-based analysis of postcranial osteology of marsupials from the Palaeocene of Itaboraí (Brazil) and the phylogenetics and biogeography of the Metatheria. Geodiversitas 20:139–302
Tarquini J, Morgan CC, Toledo N, Soibelzon LH (2019) Comparative osteology and functional morphology of the forelimb of Cyonasua (Mammalia, Procyonidae), the first South American carnivoran. J Morphol 280:446–470. doi:https://doi.org/10.1002/jmor.20956
Taylor ME (1974) The functional anatomy of the forelimb of some African Viverridae (Carnivora). J Morphol 143:307–336
Toledo N, Bargo MA, Vizcaíno SF (2013) Muscular reconstruction and functional morphology of the forelimb of early Miocene sloths (Xenarthra, Folivora) of Patagonia. Anat Rec 296:305-325. doi:https://doi.org/10.1002/ar.22627
Travouillon KJ, Legendre S, Archer M, Hand SJ (2009) Palaeoecological analyses of Riversleigh’s Oligo-Miocene sites: implications for Oligo-Miocene climate change in Australia. Palaeogeogr Palaeoclimatol Palaeoecol 276:24–37. doi:https://doi.org/10.1016/j.palaeo.2009/02.025
Walmesly A, Elton S, Louys J, Bishop LC, Meloro C (2012) Humeral epiphyseal shape in the Felidae: the influence of phylogeny, allometry, and locomotion. J Morphol 273:1424–1438. doi:https://doi.org/10.1002/jmor.20084
Warburton NM, Harvey KJ, Prideaux GJ, O’Shea JE (2011) Functional morphology of the forelimb of living and extinct tree-kangaroos (Marsupialia: Macropodidae). J Morphol 272:1230–1244. doi:https://doi.org/10.1002/jmor.10979
Wells RT, Tedford RH (1995). Sthenurus (Macropodidae, Marsupialia) from the Pleistocene of Lake Callabonna, South Australia. Bull Am Mus Nat Hist 225:1–111
Windsor D, Dagg A (1971) The gaits of the Macropodinae (Marsupialia). J Zool 163:165–175
Acknowledgements
We thank numerous museum curators for access to specimens in their care: Ross MacPhee, Rob Voss, and Eileen Westwig (Mammalogy) and Jin Meng and Judy Galkin (Vertebrate Paleontology) at the American Museum of Natural History; Hopi Hoekstra, Judy Chupasko, and Mark Omura at the Museum of Comparative Zoology; Bruce Patterson at the Field Museum of Natural History; Bill Clemens and Pat Holroyd at the University of California Museum of Paleontology; and Andrew Kitchener and Zena Timmons at the National Museum of Scotland. We are also extremely grateful for curators at Australian institutions for taking photographs of specimens of extinct kangaroos: Mary-Anne Binnie at the South Australian Museum; Tom Rich, Tim Zielger, and Hazel Richards at the National Museum of Victoria; and Kenny Travouillon at the Western Australian Museum. Thanks also to comments from two anonymous reviewers. Some of these data were collected under the auspices of Marie Curie Foundation Incoming Fellowship 623328 to CMJ. JGN was supported by the Newt and Callista Gingrich endowment.
Author information
Authors and Affiliations
Contributions
CMJ conceived of the project and collected the photographic data; AM-S and CB conducted the geometric morphological analysis; JGN conducted the analysis of humeral areas and the phylogenetic analyses, and also crafted the illustrations (with the exception of Figs. 4 and 5, by AM-S); all authors contributed to the writing of the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(XLSX 47 kb)
ESM 2
(XLSX 24 kb)
Fig. S1
Principal Components Analysis with convex hulls around the locomotor categories. Pink = suspensory, green = arboreal, blue = scansorial, black = terrestrial. Yellow stars = extant macropodids, red stars = extinct macropodids, yellow square = Pedetes capensis. (PNG 40 kb)
Fig. S2
Canonical Variates Analysis with convex hulls around the locomotor categories. Key to colors and symbols as in Fig. S1. (PNG 34 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janis, C.M., Napoli, J.G., Billingham, C. et al. Proximal Humerus Morphology Indicates Divergent Patterns of Locomotion in Extinct Giant Kangaroos. J Mammal Evol 27, 627–647 (2020). https://doi.org/10.1007/s10914-019-09494-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-019-09494-5