Abstract
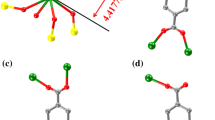
We successfully obtained two type of heterometallic complexes [Fe4Dy2(L1)2(µ4-O)2(piv)4(H2O)2(NO3)4]·CH3CN·CH3OH (1) and [Fe6Dy2(L1)2(µ4-O)2(CH3COO)6(OH)2(H2O)2(NO3)6]·CH3CN (2) by using 2,2′-((2-((2-hydroxyethyl)amino)cyclohexyl)azanediyl)bis(ethan-1-ol) (H3L1), Dy(NO3)3·6H2O, FeCl3 and pivalic acid (piv) or acetic acid under room temperature in different solvent. Crystal structure showed that the central structure of [\({{\text{Fe}}}_{4}^{{\text{III}}}\)(µ4-O)2]8+ arranged in what was often termed an absent cubic shape, although here the Fe centers were strictly coplanar. Each Fe3 triangle was connected to the capping Dy(III) through µ4-O bridges for complex 1, and each Fe3 triangle was connected to the capping Fe(III) through µ4-O bridges for complex 2. It might be due to the steric effect that the coordination atoms of piv ligands chelated and blocked the structure. AC magnetic susceptibilities exhibited that complex 1 and 2 showed out-of-phase (χ″) component of ac-susceptibilities at zero Oe static magnetic field over the frequency range of 9–999 Hz, which energy barriers were 0.44 and 0.13 K. And their energy barriers were 1.2 and 12.2 K at applied 1000 Oe static magnetic field.





Similar content being viewed by others
References
D. F. Albiol, T. A. O’Brien, W. Wernsdorfer, B. Moulton, M. J. Zaworotko, K. A. Abboud, and G. Christou (2005). Angew. Chem. Int. Ed. 44, 897.
L. M. Wittick, L. F. Jones, P. Jensen, B. Moubaraki, L. Spiccia, K. J. Berry, and K. S. Murray (2006). Dalton Trans. 12, 1534.
V. G. Makhankova, O. Y. Vassilyeva, V. N. Kokozay, J. Reedijk, G. A. van Albada, J. Jezierska, and B. W. Skelton (2002). Eur. J. Inorg. Chem 2002, 2163.
E. A. Buvaylo, V. N. Kokozay, O Yu Vassilyeva, B. W. Skelton, I. L. Eremenko, J. Jezierska, and A. Ozarowski (2009). Inorg. Chem. 48, 11092.
R. W. Saalfrank, R. Prakash, H. Maid, F. Hampel, F. W. Heinemann, A. X. Trautwein, and L. H. Böttger (2006). Chem. Eur. J. 12, 2428.
A. Scheurer, K. Gieb, M. S. Alam, F. W. Heinemann, R. W. Saalfrank, W. Kroener, K. Petukhov, M. Stocker, and P. Müller (2012). Dalton Trans. 41, 3553.
R. W. Saalfrank, C. Deutscher, H. Maid, A. M. Ako, S. Sperner, T. Nakajima, W. Bauer, F. Hampel, B. A. Hess, N. J. R. Van, R. Eikema Hommes, R. Puchta, and F. W. Heinemann (2004). Chem. Eur. J. 10, 1899.
R. W. Saalfrank, C. Deutscher, S. Sperner, T. Nakajima, A. M. Ako, E. Uller, F. Hampel, and F. W. Heinemann (2004). Inorg. Chem. 43, 4372.
J. C. Ang, Y. Mulyana, C. Ritchie, R. Clerac, and C. Boskovic (2009). Aust. J. Chem. 62, 1124.
E. M. Rumberger, S. J. Shah, C. C. Beedle, L. N. Zakharov, A. L. Rheingold, and D. N. Hendrickson (2005). Inorg. Chem. 44, 2742.
F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki, and R. A. Layfield (2018). Science 362, 1400.
C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton, and D. P. Mills (2017). Nature 548, 439.
L. Ungur, S.-Y. Lin, J. Tang, and L. F. Chibotaru (2014). Chem. Soc. Rev. 43, 6894.
J.-L. Liu, Y.-C. Chen, and M.-L. Tong (2018). Chem. Soc. Rev. 47, 2431.
K. Liu, W. Shi, and P. Cheng (2015). Coordin. Chem. Rev. 289–290, 74.
J.-L. Liu, W.-Q. Lin, Y.-C. Chen, J.-D. Leng, F.-S. Guo, and M.-L. Tong (2012). Inorg. Chem. 52, 457.
H. L. C. Feltham, R. Clérac, L. Ungur, L. F. Chibotaru, A. K. Powell, and S. Brooker (2013). Inorg. Chem. 52, 3236.
R. Sessoli and A. K. Powell (2009). Coord. Chem. Rev. 253, 2328.
S. Osa, T. Kido, N. Matsumoto, N. Re, A. Pochaba, and J. Mrozinski (2004). J. Am. Chem. Soc. 126, 420.
N. F. Chilton, S. K. Langley, B. Moubaraki, and K. S. Murray (2010). Chem. Commun. 46, 7787.
V. Mereacre, D. Prodius, Y. Lan, C. Turta, C. E. Anson, and A. K. Powell (2011). Chem. Eur. J. 17, 123.
M. N. Akhtar, V. Mereacre, G. Novitchi, J.-P. Tuchagues, Christopher E. Anson, and A. K. Powell (2009). Chem. Eur. J. 15, 7278.
C. Benelli and D. Gatteschi (2002). Chem. Rev. 102, 2369.
D. Gatteschi, R. Sessoli, and J. Villain Molecular Nanomagnets (Oxford University Press, Oxford, 2006).
G. M. Sheldrick (2015). Acta Crystallogr. Sect. C Struct. Chem. 71, 3.
Y. Yan, Y. Hu, G. P. Zhao, and X. M. Kou (2008). Dyes. Pigm. 79, 210.
Acknowledgements
This work is supported by the Special Fund for Outstanding Youth Cultivation of Henan Academy of Sciences (No. 190403004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, FW., Chen, HT., Li, DJ. et al. Two Types of {FeDy} Heterometallic Complexes Containing Fe4 Structure: Carboxylate Derivatives Effect on the Structures and Magnetic Properties. J Clust Sci 32, 461–467 (2021). https://doi.org/10.1007/s10876-020-01804-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01804-9