Abstract
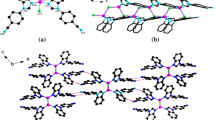
A new copper(I) iodide coordination polymer, [(CuI)3(dtb)] n (1) (dtb = 1,3-di-(1,2,4-triazole-4-yl)benzene) has been synthesized solvothermally and structurally characterized by single crystal and powder X-ray diffractions, elemental analysis, IR, and thermogravimetric analysis. Overall, 1 exhibits a 2D hybrid structure containing dtb as structure-directing agents (SDAs) and 1D Cu3I3 chain as inorganic moiety. The copper-iodide chain can be regarded as two Cu2I2 rhomboids are connected by CuI fragments via Cu–I bonds. Dtb act as bridging ligands regularly link the Cu3I3 chains along both sides through Cu–N bonds to give the final 2D network. Moreover, solid state luminescent property of 1 has been investigated at room temperature.




Similar content being viewed by others
References
M. D. Allendorf, C. A. Bauer, R. K. Bhakta, and R. J. T. Houk (2009). Chem. Soc. Rev. 38, 1330.
M. Kurmoo (2009). Chem. Soc. Rev. 38, 1353.
J. R. Li, R. J. Kuppler, and H. C. Zhou (2009). Chem. Soc. Rev. 38, 1477.
M. E. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’Keeffe, and O. M. Yaghi (2002). Science 295, 469.
S. Kitagawa, R. Kitaura, and S. Noro (2004). Angew. Chem. Int. Ed. 43, 2334.
J. J. Perry IV, J. A. Perman, and M. J. Zaworotko (2009). Chem. Soc. Rev. 38, 1400.
J. P. Zhang, Y. Y. Lin, X. C. Huang, and X. M. Chen (2005). J. Am. Chem. Soc. 127, 5495.
Y. H. Liu, J. F. Zhang, L. P. Gong, and C. Zhang (2016). J. Clust. Sci. 27, 1353.
M. A. Tershansy, A. M. Goforth, L. Peterson Jr., M. C. Burns, M. D. Smith, and H. C. zur Loye (2009). Solid State. Sci. 9, 895.
J. He, Y. G. Yin, T. Wu, D. Li, and X. C. Huang (2006). Chem. Commun. 27, 2845.
S. Hu, F. Y. Yu, Y. Yan, Z. F. Hao, L. Yu, and M. L. Tong (2011). Inorg. Chem. Commun. 14, 622.
H. H. Li, Z. R. Chen, Y. Liu, K. N. Ding, J. Q. Li, C. C. Huang, and L. Q. Guo (2007). J. Cluster. Sci. 18, 817.
T. Wu, M. Li, D. Li, and X. C. Huang (2008). Cryst. Growth. Des. 8, 568.
M. H. Bi, G. H. Li, J. Hua, Y. L. Liu, X. M. Liu, Y. W. Hu, Z. Shi, and S. H. Feng (2007). Cryst. Growth. Des. 7, 2066.
L. Maini, D. Braga, P. P. Mazzeo, L. Maschio, M. Rérat, I. Manet, and B. Ventura (2015). Dalton. Trans. 44, 13003.
J. Conesa-Egea, J. Gallardo-Martínez, S. Delgado, J. I. Martínez, J. Gonzalez-Platas, V. Fernández-Moreira, U. R. Rodríguez-Mendoza, P. Ocón, F. Zamora, and P. Amo-Ochoa (2017). Small 13, 1700965.
X. C. Shan, F. L. Jiang, D. Q. Yuan, H. B. Zhang, M. Y. Wu, L. Chen, J. Wei, S. Q. Zhang, J. Pan, and M. C. Hong (2013). Chem. Sci. 4, 1484.
M. S. Deshmukh, A. Yadav, R. Pant, and R. Boomishankar (2015). Inorg. Chem. 54, 1337.
M. A. Tershansy, A. M. Goforth, J. M. Ellsworth, M. D. Smith, and H. C. zur Loye (2008). CrystEngComm 10, 833.
H. Park, E. Kwon, H. Chiang, H. Im, K. Y. Lee, J. Kim, and T. H. Kim (2017). Inorg. Chem. 56, 8287.
A. Bonnot, C. Strohmann, M. Knorr, and P. D. Harvey (2014). J. Clust. Sci. 25, 261.
L. Li, H. Y. Li, Z. G. Ren, and J. P. Lang (2014). Eur. J. Inorg. Chem. 5, 824.
S. L. Li, J. Wang, F. Q. Zhang, and X. M. Zhang (2017). Cryst. Grwoth Des. 17, 746.
F. De Angelis, S. Fantacci, A. Sgamellotti, E. Cariati, R. Ugo, and P. C. Ford (2006). Inorg. Chem. 45, 10576.
F. S. Wu, H. B. Tong, Z. Y. Li, W. Lei, L. Liu, W. Y. Wong, W. K. Wong, and X. J. Zhu (2014). Dalton. Trans. 43, 12463.
D. Braga, F. Grepioni, L. Maini, P. P. Mazzeo, and B. Ventura (2011). New. J. Chem. 35, 339.
J. A. Tompkins, J. L. Maxwell, and E. M. Holt (1987). Inorg. Chim. Acta. 127, 1.
D. Sun, S. Yuan, H. Wang, H. F. Lu, S. Y. Feng, and D. F. Sun (2013). Chem. Commun. 49, 6152.
H. Araki, K. Tsuge, Y. Sasaki, S. Ishizaka, and N. Kitamura (2005). Inorg. Chem. 44, 9667.
W. V. Taylor, U. H. Soto, V. M. Lynch, and M. J. Rose (2016). Inorg. Chem. 55, 3206.
S. B. Miao, Z. H. Li, B. M. Ji, D. S. Deng, C. Y. Xu, and L. Zhou (2014). J. Clust. Sci. 25, 1137.
G. M. Sheldrick SHELXS-97 and SHELXL-97, Programs for Crystal Structure Refinement (University of Göttingen, Germany, 1997).
L. Yang, D. R. Powell, and R. P. Houser (2007). Dalton. Trans. 9, 955.
T. Li and S. W. Du (2008). J. Clust. Sci. 19, 323.
S. Hu and M. L. Tong (2005). Dalton. Trans. 7, 1165.
Acknowledgements
We are grateful to the Natural Science Foundation of China (Grant No. 21372112) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10876_2018_1333_MOESM1_ESM.pdf
CCDC 1583584 contains the supplementary crystallographic data for 1. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. (PDF 54 kb)
Rights and permissions
About this article
Cite this article
Miao, SB., Xu, CY., Deng, DS. et al. Synthesis, Crystal Structure, and Properties of a 2D Cu(I) Coordination Polymer Based on Cu3I3 Chains Linked by 1,3-Di-(1,2,4-Triazole-4-yl)Benzene. J Clust Sci 29, 313–317 (2018). https://doi.org/10.1007/s10876-018-1333-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1333-2