Abstract
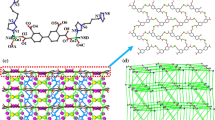
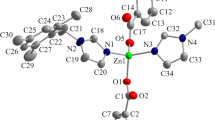
Using the solvothermal method, we present the comparative preparation of [Zn2(Hfhba)4(phen)2] (1), [Zn2(Hfhba)4(2,2′-bipy)2] (2) and [Zn2(Hfhba)4(4,4′-bipy)2]n (3), where H2fhba is 5-fluoro-2-hydroxy-benzoic acid, phen is 1,10-Phenanthroline, 2,2′-bipy is 2,2′-Bipyridine, 4,4′-bipy is 4,4′-Bipyridine. Complexes 1–3 were characterized by elemental analysis, IR, UV–Vis spectroscopy, and X-ray single-crystal diffraction. Complexes 1 and 2 are two new dinuclear complexes and 3 is a 1-D polymer. Luminescent properties of 1–3 have also been studied.








Similar content being viewed by others
References
C. S. Liu, X. S. Shi, J. R. Li, J. J. Wang, and X. H. Bu (2006). Cryst. Growth Des. 6, 656.
B. Zhao, H. L. Gao, X. Y. Chen, P. Cheng, W. Shi, D. Z. Liao, S. P. Yan, and Z. H. Jiang (2006). J. Eur. Chem. 12, 149.
S. Hu, F. Y. Yu, P. Zhang, and D. R. Lin (2013). Dalton Trans. 42, 7731.
S.-H. Zhang, Y. D. Zhang, H. H. Zou, J. J. Guo, H. P. Li, Y. Song, and H. Liang (2013). Inorg. Chim. Acta 396, 119.
J. Yang, Q. Yue, G. D. Li, J. J. Cao, G. H. Li, and J. S. Chen (2006). Inorg. Chem. 45, 2857.
B. Q. Ma, D. S. Zhang, S. Gao, T. Z. Jin, and C. H. Yan (2000). Angew. Chem. Int. Ed. 39, 3644.
R. Q. Zou, H. Sakurai, and Q. Xu (2006). Angew. Chem. Int. Ed. 45, 2542.
P. Li, H. M. Liu, X. G. Lei, X. Y. Huang, D. H. Olson, N. J. Turro, and J. Li (2003). Angew. Chem. Int. Ed. 42, 542.
J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon, and K. Kim (2000). Nature 404, 982.
W. Wang, H. Hai, S. H. Zhang, L. Yang, and C. L. Zhang (2014). J. Cluster Sci. 25, (2), 357.
S. Hu, P. Zhang, F. Y. Yu, M. X. Chen, and D. R. Lin (2014). Polyhedron 67, 388.
Q. P. Huang, G. Li, H. Y. Zhang, S.-H. Zhang, and H. P. Li (2014). Z. Anorg. Allg. Chem. 640, (7), 1403.
L. Yang, Q. P. Huang, C. L. Zhang, R. X. Zhao, and S. H. Zhang (2014). Supramol. Chem. 26, (2), 81.
D. N. Dybtsev, A. L. Nuzhdin, H. Chun, K. P. Bryliakov, E. P. Talsi, V. P. Fedin, and K. Kim (2006). Angew. Chem. Int. Ed. Engl. 45, 916.
R. Q. Zou, H. Sakurai, S. Han, R. Q. Zhong, and Q. Xu (2007). J. Am. Chem. Soc. 129, 8402.
S. Q. Ma, X. S. Wang, D. Q. Yuan, and H. C. Zhou (2008). Angew. Chem. Int. Ed. Engl. 47, 4130.
S. K. Ghosh, S. Bureekaew, and S. Kitagawa (2008). Angew. Chem. Int. Ed. Engl. 47, 3403.
R. X. Zhao, Q. P. Huang, G. Li, S. H. Zhang, H. Y. Zhang, and L. Yang (2014). J. Cluster Sci. 25, (4), 1099.
N. Guillou, C. Livage, M. Drillon, and G. Férey (2003). Angew. Chem. Int. Ed. Engl. 42, 5314.
C.-L. Zhang, X. F. Jiang, L. Yang, S. H. Zhang, and S. M. Shi (2014). J. Cluster Sci. 25, (2), 459.
J. Zhang, R. Liu, P. Y. Feng, and X. H. Bu (2007). Angew. Chem. Int. Ed. Engl. 46, 8388.
Y. Y. Liu, J. F. Ma, J. Yang, J. C. Ma, and G. J. Ping (2008). CrystEngCommun 10, 565.
L. Yang, S.-H. Zhang, W. Wang, J.-J. Guo, Q. P. Huang, R.-X. Zhao, C.-L. Zhang, and G. Muller (2014). Polyhedron 74, 49.
J. Yang, G. D. Li, J. J. Cao, Q. Yue, G. H. Li, and J. S. Chen (2007). Chem. Eur. J. 13, 3248.
M. Dinca, A. F. Yu, and J. R. Long (2006). J. Am. Chem. Soc. 128, 8904.
D. Li, T. Wu, X. P. Zhou, R. Zhou, and X. C. Huang (2005). Angew. Chem. Int. Ed. 44, 4175.
R.-X. Zhao, H. Hai, G. Li, H.-Y. Zhang, Q. P. Huang, S.-H. Zhang, and H.-P. Li (2014). J. Cluster Sci.. doi:10.1007/s10876-014-0750-0.
S. H. Zhang, L. F. Ma, H. H. Zou, Y. G. Wang, H. Liang, and M. H. Zeng (2011). Dalton Trans. 40, 11402.
S. H. Zhang, R. X. Zhao, H. P. Li, C. M. Ge, Q. P. Huang, and H. H. Zou (2014). J. Solid State Chem. 216, 30.
G. Li, W. Wang, S. H. Zhang, H. Y. Zhang, and F. Y. Chen (2014). J. Cluster Sci.. doi:10.1007/s10876-014-0758-5.
R. A. Reynolds III, W. O. Yu, W. R. Dunham, and D. Coucouvanis (1996). Inorg. Chem. 35, 2721.
S. H. Zhang, N. Li, C. M. Ge, C. Feng, and L. F. Ma (2011). Dalton Trans. 40, 3000.
G. M. Sheldrick (2008). Acta Cryst. A64, 112.
S.-H. Zhang, C.-L. Lan, and Y.-M. Jiang (2004). Chin. J. Struct. Chem. 23, (8), 878.
H.-F. Xu, S.-H. Zhang, Y.-M. Jiang, X.-X. Zhong, and F. Gao (2004). Chin. J. Struct. Chem. 23, (7), 808.
Z. Shi, G. H. Li, L. Wang, L. Gao, X. B. Chen, J. Hua, and S. H. Feng (2004). Cryst. Grow. & Des. 4, (1), 25.
L.-M. Zheng, X. Q. Wang, Y. S. Wang, and A. J. Jacobson (2001). J. Mater. Chem. 11, 1100.
X.-M. Chen, Y.-X. Tong, and T. C. W. Mak (1994). Inorg. Chem. 33, (20), 4586.
D. V. Soldatov, P. Tinnemans, G. D. Enright, C. I. Ratcliffe, P. R. Diamente, and J. A. Ripmeester (2003). Chem. Mater. 15, 3826.
S. L. Zheng and X. M. Chen (2004). Aust. J. Chem. 57, 703.
B. Xiao, H. W. Hou, and Y. T. Fan (2009). J. Coord. Chem. 62, 1827.
M. Shebl (2014). Spectrochim. Acta A. 117, 127.
K. Nakamoto Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed (John Wiley and Sons, New York, 1997).
S. Mishra, S. Daniele, G. Ledoux, E. Jeanneau, and M. F. Joubert (2010). Chem. Commun. 46, 3756.
L. E. Valenti, M. B. Paci, C. P. D. Pauli, and C. E. Giacomelli (2011). Anal. Biochem. 410, 118.
S. H. Zhang, Y. M. Jiang, and X. X. Zhong (2004). Chin. J. Inorg. Chem. 20, 959.
Acknowledgments
This work is financially supported by Startup foundation for Dr Xiao of Guilin University of Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10876_2014_799_MOESM1_ESM.docx
CCDC 1013842–1013844 contains the supplementary crystallographic data for complex 1–3. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Electronic Supplementary Information (ESI) available: [Structure of 2, Packing drawing of 1 and 3.]
Rights and permissions
About this article
Cite this article
Xiao, Y., Huang, P. & Wang, W. Ligand Structure Induced Diversification from Dinuclear to 1D Chain Compounds: Syntheses, Structures and Fluorescence Properties. J Clust Sci 26, 1091–1102 (2015). https://doi.org/10.1007/s10876-014-0799-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0799-9