Abstract
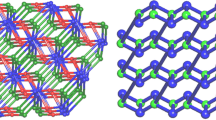
Hydrothermal reactions of 1, 2, 4, 5-benzenetetracarboxylic acid (H4BTC) with cadmium acetate and copper acetate in the presence of imidazole (imz) resulted in two new coordination polymers, namely, [Cd(imz)3]2(BTC)·0.5H2O 1, [Cu4(H2O)2(imz)8](BTC)2·7H2O 2. Two compounds have been characterized structurally using single-crystal diffraction, elemental analysis, and FT-IR spectrum. In compound 1, the hexa-coordinated Cd(II) ions are bridged by BTC to form two-dimensional rhombohedral grid sheets. In compound 2, the coordination polyhedrons [CuO4N2] and [CuO3N2] are bridged by BTC to lead to a 2D framework with rectangular-shaped cavities. Two compounds of third-order nonlinear optical (NLO) properties were determined by Z-scan technique in DMSO solution. The results showed that two compounds exhibited strong NLO absorption and strong self-focusing effects. The third-order NLO absorptive coefficients β(MKS) are 5.45 × 10−11 m W−1 for 1 and 9.81 × 10−11 m W−1 for 2. The refractive indexes γ(MKS) are 3.96 × 10−18 m2 W−1 for 1 and 7.60 × 10−18 m2 W−1 for 2. The third-order NLO susceptibility χ(3) are calculated to be 1.25 × 10−11 for 1, 2.32 × 10−11 esu for 2, respectively. These values are larger than those of metal coordination polymers reported.
Graphical Abstract
Two novel two-dimensional transition metal coordination polymers have been reported. Two compounds exhibit strong NLO absorption and strong self-focusing effects. The third-order NLO susceptibility χ(3) are calculated to be 1.25 × 10−11 for 1, 2.32 × 10−11 esu for 2, respectively






Similar content being viewed by others
References
K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae, and J. R. Long (2012). Chem. Rev. 112, 724.
H. H. Wu, Q. H. Gong, D. H. Olson, and J. Li (2012). Chem. Rev. 112, 836.
M. Y. Yoon, R. Srirambalaji, and K. Kim (2012). Chem. Rev. 112, 1196.
J. R. Li, J. L. Sculley, and H. C. Zhou (2012). Chem. Rev. 112, 869.
L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne, and J. T. Hupp (2012). Chem. Rev. 112, 1105.
W. Zhang and R. G. Xiong (2012). Chem. Rev. 112, 1163.
M. P. Suh, Y. J. Par, T. K. Prasad, and D. W. Lim (2012). Chem. Rev. 112, 782.
Y. J. Cui, Y. F. Yue, G. D. Qian, and B. L. Chen (2012). Chem. Rev. 112, 1126.
J. P. Zhang, Y. B. Zhang, J. B. Lin, and X. M. Chen (2012). Chem. Rev. 112, 1001.
M. O’Keeffe and O. M. Yaghi (2012). Chem. Rev. 112, 675.
C. Wang, T. Zhang, and W. B. Lin (2012). Chem. Rev. 112, 1084.
N. Stock and S. Biswas (2012). Chem. Rev. 112, 933.
H. Furukawa, K. E. Cordova, M. O’Keeffe, and O. M. Yaghi (2013). Science 341, 974.
J. P. Zhou, Q. Peng, Z. H. Wen, G. S. Zeng, Q. J. Xing, and G. G. Guo (2010). Cryst. Growth Des. 10, 2613.
H. W. Hou, Y. L. Wei, Y. L. Song, Y. Zhu, L. K. Li, and Y. T. Fan (2002). J. Mater. Chem. 12, 838.
Y. Y. Niu, Y. L. Song, T. N. Chen, Z. L. Xue, and X. Q. Xin (2001). CrystEngComm. 3, 152.
H. W. Hou, X. R. Meng, Y. L. Song, Y. T. Fan, Y. Zhu, H. J. Lu, C. X. Du, and W. H. Shao (2002). Inorg. Chem. 41, 4068.
B. Sui, W. Zhao, G. H. Ma, T. A. Okamura, J. Fan, Y. Z. Li, S. H. Tang, W. Y. Sun, and N. Ueyama (2004). J. Mater. Chem. 14, 1631.
Y. Wang and L. T. Cheng (1992). J. Phys. Chem. 96, 1530.
H. W. Hou, H. G. Ang, S. G. Ang, Y. T. Fan, M. K. W. Low, W. Ji, and Y. W. Lee (1999). Phys. Chem. Chem. Phys. 1, 3145.
G. M. Sheldrick, G. M(2008), Acta Cryst. A64, 112.
Q. Hua, Y. Zhao, G. C. Xu, M. S. Chen, Z. Su, K. Cai, and W. Y. Sun (2010). Cryst. Growth Des. 10, 2553.
Y. J. Mu, G. Han, S. Y. Ji, H. W. Hou, and Y. T. Fan (2011). CrystEngComm. 13, 5943.
B. Tao, H, Xia, C. X. Huang, X. W. Li (2011), Z. Anorg. Allg. Chem. 637, 703.
G. H. Cui, C. H. He, C. H. Jiao, J. C. Geng, and V. A. Baltov (2012). CrystEngComm. 13, 4210.
Y. Y. Liu, H. Y. Liu, J. F. Ma, Y. Yang, and J. Yang (2013). CrystEngComm. 15, 1897.
L. Ma, N. Q. Yu, S. S. Chen, and H. Deng (2013). CrystEngComm. 15, 1352.
S. Y. Niu, Y. X. Chi, J. Jin, G. D. Yang, and L. Ye (2006). Struct. Chem. 17, 209.
L. L. Wen, J. B. Zhao, K. L. Lv, K. J. Deng, X. K. Leng, and D. F. Li (2012). Cryst. Growth Des. 12, 1603.
E. C. Yang, B. Ding, Z. Y. Liu, Y. L. Yang, and X. J. Zhao (2012). Cryst. Growth Des. 12, 1185.
M. S. Bahae, A. A. Said, T. H. Wei, D. J. Hagan, and E. W. V. Stryland (1990). IEEE J. Quantum Electron. 26, 760.
M. S. Bahae, A. A. Said, and E. W. V. Stryland (1989). Opt. Lett. 14, 955.
J. L. Zhou, Q. Y. Chen, Y. Y. Gu, and G. Q. Mei (2005). Transition Met. Chem. 30, 1036.
Acknowledgments
The work was financially supported by Natural Science Fund Project of Education Department of Henan Province (Grant Nos.13A150293 and 14A150023).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, C., Feng, X., Zhao, N. et al. Syntheses, Structures and Third-Order Nonlinear Optical Properties of Two-Dimensional Rhombohedral Grid Coordination Polymers: [Cd(imz)3]2(BTC)·0.5H2O and [Cu4(H2O)2(imz)8](BTC)2·7H2O(BTC = 1, 2, 4, 5-benzenetetracarboxylate anion, imz = imidazole). J Clust Sci 26, 889–900 (2015). https://doi.org/10.1007/s10876-014-0778-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0778-1