Abstract
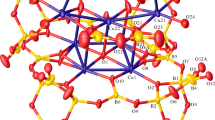
The syntheses and structures of [K(18-crown-6)]2[K(18-crown-6)(H2O)2]2[Nb6Cl12(CN)6] · 2CH3CN (1) and [(C6H5)4P]4[Nb6Cl12(NCS)6] · 0.94CH3OH (2), determined by X-ray single crystal diffraction, are reported. Crystal data: [K(18-crown-6)]2[K(18-crown-6)(H2O)2]2[Nb6Cl12(CN)6] · 2CH3CN: monoclinic, P21/n, a = 17.8240(9), b = 15.9395(8), c = 18.660(1) Å, β = 113.833(2)°, Z = 2; [(C6H5)4P]4[Nb6Cl12(NCS)6] · 0.94CH3OH: triclinic, \( P\bar{1} \), a = 14.6239(3), b = 14.6237(5), c = 15.9831(3) Å, α = 113.482(1)°, β = 114.684(1)°, γ = 92.585(1)°, Z = 1. Both complexes contain [Nb6Cl12 Y 6]4− cluster anions with Y = CN and NCS, respectively, on all six cluster exo-positions. They have been prepared via ligand substitution in solution, starting from K4[Nb6Cl18], which was synthesized by a high temperature solid state reaction. Structural trends and spectroscopic properties are discussed and compared to related compounds reported previously in the literature.






Similar content being viewed by others
References
P. A. Vaughan, J. H. Sturdivant, and L. Pauling (1950). J. Am. Chem. Soc. 72, 5477.
A. Simon, H. G. von Schnering, H. Wöhrle, and H. Schäfer (1965). Z. Anorg. Allg. Chem. 339, 155.
H. Schäfer and H. G. von Schnering (1964). Angew. Chem. 20, 833.
A. Simon (1988). Angew. Chem. 100, 163.
A. Simon (1988). Angew. Chem. Int. Ed. 27, 159.
G. Meyer (1988). Chem. Rev. 88, 93.
J. D. Corbett, in E. Parthé (ed.), Modern Perspectives in Inorganic Crystal Chemistry (Kluwer, Dortrecht, the Netherlands, 1992), p. 27.
A. Simon, in G. Schmidt (ed.), Clusters and Colloids. From Theory to Applications (VCH Publishers, Weinheim, 1994), Chapter 5, p. 373.
J. R. Long, L. S. McCarty, and R. H. Holm (1996). J. Am. Chem. Soc. 118, 4603.
T. Saito (1999). J. Chem. Soc. Dalton Trans. 2, 97, and references therein.
N. Prokopuk and D. F. Shriver (1999). Adv. Inorg. Chem. 46, 1.
C. Perrin, in P. Braunstein, L. A. Oro, and P. R. Raithby (eds.), Metal Clusters in Chemistry, Vol. 3 (Wiley, Weinheim, 1999), p. 1563.
J. D. Corbett (2000). Inorg. Chem. 39, 5178, and references therein.
W. C. Dorman and R. E. McCarley (1974). Inorg. Chem. 13, 491.
A. Simon, H. G. von Schnering, and H. Schäfer (1968). Z. Anorg. Allg. Chem. 361, 235.
F. W. Koknat, J. A. Parsons, and A. Vongvusharintra (1974). Inorg. Chem. 13, 1699.
P. B. Fleming, L. A. Müller, and R. E. McCarley (1967). Inorg. Chem. 6, 1.
A. Broll, D. Juza, and H. Schäfer (1971). Z. Anorg. Allg. Chem. 382, 69.
A. Broll and H. Schäfer (1970). J. Less-Common Met. 22, 367.
C. Perrin and M. Sergent (1991). J. Solid State Chem. 28, 933.
S. Ihmaïne, C. Perrin, O. Peña, and M. Sergent (1988). J. Less-Common Met. 137, 323.
O. Peña, S. Ihmaïne, C. Perrin, and M. Sergent (1990). Solid State Commun. 74, 285.
S. Ihmaïne, C. Perrin, O. Peña, and M. Sergent (1990). Physica B163, 615.
C. Perrin, S. Cordier, S. Ihmaïne, and M. Sergent (1995). J. Alloys Compd. 229, 123.
S. Ihmaïne, C. Perrin, and M. Sergent (1987). Acta Crystallogr. 43C, 813.
S. Ihmaïne, C. Perrin, and M. Sergent (1989). Acta Crystallogr. 45C, 705.
T. Duraisamy, J. S. Qualls, and A. Lachgar (2003). J. Solid State Chem. 170, 227.
A. Nägele, E. Anokhina, J. Sitar, H.-J. Meyer, and A. Lachgar (2000). Z. Naturforsch. 55B, 139.
B. Baján and H.-J. Meyer (1995). Z. Naturforsch. 50B, 1373.
A. Lachgar and H.-J. Meyer (1994). J. Solid State Chem. 110, 15.
N. Brničević, B. Kojić-Prodić, and D. Plavšić (1981). Z. Anorg. Allg. Chem. 472, 200.
H. Schäfer, B. Plautz, and H. Plautz (1972). Z. Anorg. Allg. Chem. 392, 10.
F. W. Koknat, J. A. Parsons, and A. Vongvusharintra (1974). Inorg. Chem. 13, 1699.
B. Spreckelmeyer (1968). Z. Anorg. Allg. Chem. 358, 147.
D. D. Klendworth and R. A. Walton (1981). Inorg. Chem. 20, 1151.
H. Imoto, S. Hayakawa, N. Morita, and T. Saito (1990). Inorg. Chem. 29, 2007.
U. Beck, A. Simon, N. Brničević, and S. Širac (1995). Croat. Chem. Acta 68, 837.
B. Yan, H. Zhou, and A. Lachgar (2003). Inorg. Chem. 42, 8818.
N. G. Naumov, S. Cordier, and C. Perrin (2002). Angew. Chem. 114, 3128.
N. G. Naumov, S. Cordier, and C. Perrin (2002). Angew. Chem. Int. Ed. 41, 3002.
A. Bernsdorf, A.Flemming, and M. Köckerling, unpublished results.
N. G. Naumov, S. Cordier, and C. Perrin (2003). Solid State Sci. 5, 1359.
N. G. Naumov, S. Cordier, and C. Perrin (2004). Chem. Commun. 1126.
O. Reckeweg and H.-J. Meyer (1996). Z. Anorg. Allg. Chem. 622, 411.
H.-J. Meyer (1995). Z. Anorg. Allg. Chem. 621, 921.
O. Reckeweg and H.-J. Meyer (1995). Z. Naturforsch. 50b, 1377.
O. Reckeweg, H.-J. Meyer, and A. Simon (2002). Z. Anorg. Allg. Chem. 628, 920.
L. F. Piedra-Garza and M. Köckerling (2006). Inorg. Chem. 45, 8829.
U. Beck, A. Simon, N. Brničević, and S. Širac (1995). Croat. Chem. Acta 68, 837.
B. Yan, C. S. Day, and A. Lachgar (2004). Chem. Commun. 2390.
H. Zhou, K. C. Strates, M. Á. Muñoz, K. J. Little, D. M. Pajerowski, M. W. Meisel, D. R. Talham, and A. Lachgar (2007). Chem. Mater. 19, 2238.
J.-J. Zhang, H.-J. Zhou, and A. Lachgar (2007). Angew. Chem. 119, 5083,
J.-J. Zhang, H.-J. Zhou, and A. Lachgar (2007). Angew. Chem. Int. Ed. 46, 4995.
H. Zhou, C. S. Day, and A. Lachgar (2004). Chem. Mater. 16, 4870.
H. Zhou, C. S. Day, and A. Lachgar (2006). Cryst. Growth Des. 6, 2384.
Y. Kim, S. M. Park, W. Nam, and S. J. Kim (2001). Chem. Commun. 1470.
Y. Kim, S. M. Park, and S. J. Kim (2002). Inorg. Chem. Commun. 5, 592.
H. Zhou and A. Lachgar (2007). Eur. J. Inorg. Chem. 1053.
Z. Yan, C. S. Day, and A. Lachgar (2005). Inorg. Chem. 44, 4499.
N. Prokopuk and D. F. Shriver (1999). Chem. Mater. 11, 1230.
U. Welz-Biermann, N. V. Ignatiev, E. Bernhardt, M. Finze, and H. Willner (2004). Merck Patent GmbH, Darmstadt, WO 2004/072089.
SADABS, Diffractometer Absorption- and Scaling Program (Bruker-Nonius Inc, Madison, WI, USA, 2003).
G. M. Sheldrick, SHELXS97 and SHELXL97 (Programs for the Solution and Refinement of Crystal Structures, Göttingen 1997).
N. G. Naumov, S. Cordier, F. Gulo, T. Roisnel, V. E. Fedorov, and C. Perrin (2003). Inorg. Chim. Acta 350, 503.
P. M. Boorman and B. P. Straughan (1966). J. Chem. Soc.(A), 1514.
R. Mattes (1969). Z. Anorg. Allg. Chem. 364, 297.
Acknowledgments
We thank Dipl.-Chem. Alexander Wulf and Prof. Dr. Ralf Ludwig for the FIR spectra and Prof. Dr. Helmut Reinke for maintaining the X-ray equipment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dr. Christiane Perrin and Professor Dr. André Perrin.
Rights and permissions
About this article
Cite this article
Flemming, A., Bernsdorf, A. & Köckerling, M. New Cluster Complexes with Octahedral Cores of Niobium Atoms: Syntheses, Structures, and Properties of [K(18-crown-6)]2[K(18-crown-6)(H2O)2]2[Nb6Cl12(CN)6] · 2CH3CN and [(C6H5)4P]4[Nb6Cl12(NCS)6] · 0.94CH3OH. J Clust Sci 20, 113–131 (2009). https://doi.org/10.1007/s10876-008-0217-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-008-0217-2