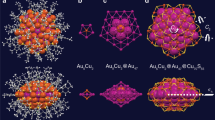
As part of an extensive effort to synthesize a variety of nanosized gold–palladium carbonyl phosphine clusters, the neutral Au4Pd32(CO)28(PMe3)14 (1) was isolated and unambiguously characterized by low-temperature CCD X-ray diffraction and IR measurements. This nanosized Au4Pd32 cluster was prepared in low yields (<5%) from the room-temperature reaction of Pd10(CO)12(PMe3)6 (2) with Au(SMe2)Cl in THF/acetone. The heretofore unknown molecular geometry of 1 of pseudo-D2 (222) symmetry (without methyl substituents) may be viewed to arise from a relatively strong (Au–Au)-bonded linkage (2.64 Å (av)) of two pentagonal-bipyramidal (μ5-Au)(μ5-Pd)Pd5 polyhedra; this generated 14-atom Au2Pd12 unit may be considered as a markedly deformed part of a 19-atom Au-centered double icosahedron without the inner pentagon (corresponding to five missing inner atoms). In turn, two Au2Pd12 units form a central composite-twinned Au4Pd22 kernel via vertex-fusion of two common Pd atoms along with additional formation of four Pd–Pd bonding, four Au–Pd bonding, and two weaker secondary Au–Au bonding interactions at 2.90 Å (av) (versus the other two diagonal Au–Au nonbonding ones at 3.51 Å (av)); this resulting Au4Pd22P8 kernel is augmented by the addition of two triangular Pd3P core-fragments and four exopolyhedral PdP groups to give the Au4Pd32P14 framework of 1. This cluster is stabilized by 28 bridging COs, of which 20 are doubly bridging and 8 triply bridging. The largest metal-core diameter of 1 along one pseudo C2 axis is 1.1 nm. This new type of multi-twinned metal cluster has direct relevance to both ligated and non-ligated (naked) non-crystalline metal nanoparticles, many of which possess multiple twinning and/or disorder.
Similar content being viewed by others
References
(a) J. H. Sinfelt (1983). Bimetallic Catalysts (Wiley: New York) Chap. 1 and 2. (b) I.E.Wacks (1983). Gold Bull. 16, 98. (c) J. Schwank (1985). Gold Bull. 18, 1. (d) B. D. Alexander, M. P. Gomez-Sal, P. R. Gannon, C. A. Blaine, P. D. Boyle, A. M. Mueting, and L. H. Pignolet (1988). Inorg. Chem. 27, 3301. (e) A. M. Mueting, W. Bos, B. D. Alexander, P. D. Boyle, J. A. Casalnuovo, S. Balaban, L. N. Ito, S. M. Johnson, and L. H. Pignolet (1988). New J. Chem. 12, 505. (f) L. H. Pignolet, M. A. Aubart, K. L. Craighead, R. A. T. Gould, D. A. Krogstad, and J. S. Wiley (1995). Coord. Chem. Rev. 143, 219. (g) D.A.Krogstad, W. V. Konze, and L. H. Pignolet (1996). Inorg. Chem. 35, 6763. (h) P. G. Jones (1986). Gold Bull. 19, 46. (i) K. P. Hall and D. M. P. Mingos (1984). Progr. Inorg. Chem. 32, 237. (j) N. Toshima and T. Yonezawa (1998). New J. Chem. 22, 1179. (k) I.T.Horvath (2003). Encyclopedia of Catalyses (Wiley-Interscience), Vol. 1, 621–665. (l) P.Braunstein and J. Rose, in I. Bernal (ed.), Stereochemistry of Organometallic and Inorganic Compounds (Elsevier: Tarrytown, NY, 1998), Vol. 3. (m) M. Ichikawa (1992). Adv. Catal. 38, 283 and references therein. (n) Catalysis by Di- and Polynuclear Metal Cluster Complexes; R. D. Adams, F. A. Cotton (eds.), (Wiley-VCH: New York, 1998). (o) D. M. P. Mingos and M. J. Watson (1992). Adv. Inorg. Chem. 39, 327, and references therein
(a) L. I. Rubenstein and L. H. Pignolet (1996). Inorg. Chem. 35, 6755. (b) D. M. P. Mingos and M. J. Watson (1992). Adv. Inorg. Chem. 39, 327. (c) K. L. Craighead, A. M. P. Felicissimo, D. A. Krogstad, L. T. J. Nelson, and L. H. Pignolet (1993). Inorg. Chim. Acta. 212, 31, and references therein. (d) N. H. Takata, A. M. P. Felicissimo, and V. G. Young, Jr. (2001). Inorg. Chim. Acta. 325, 79, and references therein. (e) A. D. Burrows, A.A.Gosden, C. M. Hill, and D. M. P. Mingos (1993). J. Organometal. Chem. 452, 251. (f) S.-M. Lee and W.-T. Wong (1998). J. Cluster Sci. 9, 417, and references therein. (g) J. Strähle, in P. Braunstein, L. A. Oro, and P. R. Raithby (eds.),Metal Clusters in Chemistry (Wiley-VCH, New York, 1999) Vol. 1
(a) L. N. Ito, B. J. Johnson, A. M. Mueting, and L. H. Pignolet (1989). Inorg. Chem. 28, 2026. (b) L. N. Ito, A. M. P. Felicissimo, and L. H. Pignolet (1991). Inorg. Chem. 30, 988
(a) M. Laupp and J. Strähle (1995). Z. Naturforsch., Teil B, 50, 1369. (b) M. Laupp and J. Strähle (1994). Angew. Chem., Int. Ed. Engl. 33, 207
R. Copely C. M. Hill D. M. P. Mingos (1995) J. Cluster Sci 6 71 Occurrence Handle10.1007/BF01175837
(a) N. Toshima, M. Harada, Y. Yamazaki, and K. Asakura (1992). J. Phys. Chem. 96, 9927. (b) T. Yonezawa and N. Toshima (1995). J. Chem. Soc., Faraday Trans. 91, 4111. (c) N. Toshima and T. Yonezawa (1998). New J. Chem. 22, 1179
N. T. Tran M. Kawano D. R. Powell R. K. Hayashi C. F. Campana L. F. Dahl (1999) J. Am. Chem. Soc 121 5945 Occurrence Handle10.1021/ja982637c Occurrence Handle1:CAS:528:DyaK1MXjs1Kjt70%3D
G. J. Miller (1998). Eur. J. Inorg. Chem. 5, 523 and references therein
(a) L. Pasteur (1848). Ann. Chim. Phys. 24, 442. (b) G. B. Kauffman and R. D. Meyers (1975). J. Chem. Ed. 52, 777
N. T. Tran and L. F. Dahl (2005). Manuscript in preparation
N. T. Tran, D. R. Powell, and L. F. Dahl (2004). J. Chem. Soc., Dalton Trans. 209
(a) E. G. Mednikov, S. A. Ivanov, J. Wittayakun, and L. F. Dahl (2003). J. Chem. Soc., Dalton Trans. 1686. (b) E. G. Mednikov, J. Wittayakun, and L. F. Dahl (2005). Manuscript in preparation
N. T. Tran, D. R. Powell, and L. F. Dahl (2004). J. Chem. Soc., Dalton Trans. 217
N. T. Tran, M. Kawano, and L. F. Dahl (2001). J. Chem. Soc., Dalton Trans. 2731
(a) A. J. Whoolery and L. F. Dahl (1991). J. Am. Chem. Soc. 113, 6683. (b) A. J. Whoolery-Johnson, B. Spencer, and L. F. Dahl (1994). Inorg. Chim Acta. 227, 269
(a) D. M. P. Mingos (1983). J. Chem. Soc., Chem. Commun. 706. (b) D. M. P. Mingos (1983). J. Chem. Soc., Chem. Commun. 1352. (c) M. P. Mingos (1984). Acc. Chem. Res. 17, 311. (d) D. M. P. Mingos (1984), Polyhedron, 3, 1289. (e) K. P. Hall and D. M. P. Mingos (1984). Progr. Inorg. Chem. 32, 237. (f) D. M. P. Mingos and R. L. Johnson (1985). J. Organomet. Chem. 281, 419. (g) D. M. P. Mingos and L. Zhenyang (1988). J. Chem. Soc., Dalton Trans. 1657 and references therein; (h) D. M. P. Mingos and A. P. May (1990). in D.F. Shriver, H.D. Kaesz and R. D. Adams (eds.), The Chemistry of Metal Cluster Complexes, (VCH Publishers, New York), chap. 2, pp.11–119; (i) D. M. P. Mingos and D. J. Wales (1990). Introduction to Cluster Chemistry, (Prentice Hall, Old Tappan, NJ)
(a) B. K. Teo and H. Zhang (1990). Polyhedron. 9, 1985. (b) B. K. Teo and N. J. Sloane (1986). Inorg. Chem. 25, 2315
G. Ciani A. Sironi (1980) J. Organomet. Chem 197 233 Occurrence Handle10.1016/S0022-328X(00)93569-1 Occurrence Handle1:CAS:528:DyaL3MXjs1OitA%3D%3D
E. G. Mednikov and N. K. Eremenko (1982). Izv. Acad. Nauk SSSR. Ser. Khim. 2540. [Bull. Acad. Sc. USSR, Div. Chem. Sc., 1982, 31, 2240 (Engl. Transl.)]
G. Sheldrick: all crystallographic software and sources of the scattering factors are contained in the SHELXTL (version 6.10 (2000)) program library, Bruker Analytical X-Ray Systems, Madison, WI
Acknowledgments
This research was supported by the National Science Foundation. Departmental purchase of a CCD area detector system was made possible by funds from NSF, the UW-Madison graduate school, and the chemistry department. Color drawings were prepared with Crystal Maker, Interactive Crystallography. David C. Palmer (P.O. Box 183 Bicester, Oxfordshire, UK OXG TBS).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to Professor F. A. Cotton on the occasion of his 75th birthday in recognition of numerous seminal contributions to modern Inorganic Chemistry. Professor Cotton has a truly unparalleled scientific career in Inorganic Chemistry in terms of the overall composite effects of his highly prolific research productivity, his tremendous impact on former graduate students, postdoctoral associates, and collaborators, and his matchless textbooks/monographs.
Rights and permissions
About this article
Cite this article
Mednikov, E.G., Dahl, L.F. Nanosized Au4Pd32(CO)28(PMe3)14 Containing a Highly Distorted Encapsulated Au4 Tetrahedron: Proposed Multi-Twinned Growth-Pattern from Two Deformed Au-Centered Double Icosahedral-Based Fragments*. J Clust Sci 16, 287–302 (2005). https://doi.org/10.1007/s10876-005-4969-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10876-005-4969-7