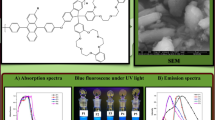
Abstract
A series of high molecular weight fluorene-based soluble poly(arylene ethynylene)s (PAEs) have been prepared and characterized. The polymers consist of 2,5-bis(3-tetradecylthiophen-2-yl)-3a,6a-dihydrothieno[3,2-b]thiophene, 2,5-bis(3-tetradecylthiophen-2-yl)-3a,6a-dihydrothiazolo[5,4-d]thiazole, or 4,7-bis(3-tetradecylthiophen-2-yl)benzo[c] [1, 2, 5] thiadiazole unit with an electron donor 9,9-bis(2-ethylhexyl)-9H-fluorene unit connected via electron accepting ethynylene linkage. The molecular weights (M w) of the polymers were found to be in the range of 103600–179000 g/mol with polydispersity index (PDI) of 3.9–5.0. Optical and redox properties have been investigated by UV–visible, fluorescence spectroscopy, and cyclic voltammetry (CV) measurements. Combination of experimental and density functional theory (DFT) calculations indicated that the benzothiadiazole unit incorporated polymer has lowest band gap with most stable lowest unoccupied molecular orbital (LUMO) energy level. Polymer light emitting diode properties have been investigated for the polymer having highest molecular weight with device configuration ITO/PEDOT:PSS/Polymer/LiF/Al. Well-behaved diode characteristics with EL maxima at 600 nm were observed.
Graphical Abstract










Similar content being viewed by others
References
Sirringhaus H (2014) 25th Anniversary article: organic field-effect transistors: the path beyond amorphous silicon. Adv Mater 26:1319–1335
Palai AK, Lee J, Jea M, Hanah Na H, Shin TJ, Jang S, Park S-U, Pyo S (2014) Symmetrically functionalized diketopyrrolopyrrole with alkylated thiophene moiety: from synthesis to electronic devices applications. J Mater Sci 49:4215–4224
Grimsdale AC, Chan K, Martin RE, Jokisz PG, Holmes AB (2009) Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem Rev 109:897–1091
Palai AK, Kumar A, Shashidhara KK, Mishra SP (2014) Polyalkylthiophene-containing electron donor and acceptor heteroaromatic bicycles: synthesis, photo-physical, and electroluminescent properties. J Mater Sci 49:2456–2464
Liu J, Durstockb M, Dai L (2014) Graphene oxide derivatives as hole- and electron-extraction layers for high-performance polymer solar cells. Energy Environ Sci 7:1297–1306
Gao J, Chen W, Dou L, Chen C-C, Chang W-H, Liu Y, Li G, Yang Y (2014) Elucidating double aggregation mechanisms in the morphology optimization of diketopyrrolopyrrole-based narrow bandgap polymer solar cells. Adv Mater 26:3142–3147
Balamurugan A, Reddy MLP, Jayakannan M (2013) π-Conjugated polymer-Eu3+ complexes: versatile luminescent molecular probes for temperature sensing. J Mater Chem A 1:2256–2266
Niu Q, Gao K, Lin Z, Wu W (2013) Surface molecular-imprinting engineering of novel cellulose nanofibril/conjugated polymer film sensors towards highly selective recognition and responsiveness of nitroaromatic vapors. Chem Commun 49:9137–9139
Bolduc A, Skene WG (2014) Direct preparation of electroactive polymers on electrodes and their use in electrochromic devices. Polym Chem 5:1119–1123
Osterholm AM, Shen DE, Dyer AL, Reynolds JR (2013) Optimization of PEDOT films in ionic liquid supercapacitors: demonstration as a power source for polymer electrochromic devices. ACS Appl Mater Interfaces 5(24):13432–13440
Krebs FC, Jørgensen M, Norrman K, Hagemann O, Alstrup J, Nielsen TD, Fyenbo J, Larsen K, Kristensen J (2009) A complete process for production of flexible large area polymer solar cells entirely using screen printing-first public demonstration. Sol Energy Mater Sol Cells 93:422–441
Sandstorm A, Dam HF, Krebs FC, Edman L (2012) Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating. Nat Commun 3:1002. doi:10.1038/ncomms2002
Egbe DAM, Neugebauer H, Sariciftci NS (2011) Alkoxy-substituted poly(arylene-ethynylene)-alt-poly(arylene-vinylene)s: synthesis, electroluminescence and photovoltaic applications. J Mater Chem 21:1338–1349
Dallos T, Beckmann D, Brunklaus G, Baumgarten M (2011) Thiadiazoloquinoxaline-acetylene containing polymers as semiconductors in ambipolar field effect transistors. J Am Chem Soc 133:13898–13901
Thomas SW III, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386
Zheng J, Swager TM (2005) Poly(arylene ethynylene)s in chemosensing and biosensing. Adv Polym Sci 177:151–179
Davey AP, Elliott S, O’Conner O, Blau W (1995) New rigid backbone conjugated organic polymers with large fluorescence quantum yields. J Chem Soc, Chem Commun 14:1433–1434
Bunz UHF (2000) Poly(aryleneethynylene)s: syntheses, properties, structures, and applications. Chem Rev 100:1605–1644
Tekin E, Egbe DAM, Kranenburg JM, Ulbricht C, Rathgeber S, Birckner E, Rehmann N, Meerholz K, Schubert US (2008) Effect of side chain length variation on the optical properties of PPE-PPV hybrid polymers. Chem Mater 20:2727–2735
Guo X, Watson MD (2011) Pyromellitic diimide-based donor-acceptor poly(phenylene ethynylene)s. Macromolecules 44:6711–6716
Breen CA, Tischler Y, Bulovic V, Swager TM (2005) Highly efficient blue electroluminescence from poly(phenylene ethynylene) via energy transfer from a hole-transport matrix. Adv Mater 17:1981–1985
Itskos G, Xristodoulou X, Iliopoulos E, Ladas S, Kennou S, Neophytou M, Choulis S (2013) Electronic and interface properties of polyfluorene films on GaN for hybrid optoelectronic applications. Appl Phys Lett 102:063303 (1–5)
Pogantsch A, Wenzl FP, List EJW, Leising G, Grimsdale AC, Müllen K (2002) Polyfluorenes with dendron side chains as the active materials for polymer light-emitting devices. Adv Mater 14:1061–1064
Watters DC, Yi H, Pearson AJ, Kingsley J, Iraqi A, Lidzey D (2013) Fluorene-based co-polymer with high hole mobility and device performance in bulk heterojunction organic solar cells. Macromol Rapid Commun 34:1157–1162
Zheng H, Zheng Y, Liu N, Ai N, Wang Q, Wu S, Zhou J, Hu D, Yu S, Han S, Xu W, Luo C, Meng Y, Jiang Z, Chen Y, Li D, Huang F, Wang J, Peng J, Cao Y (2013) All-solution processed polymer light-emitting diode displays. Nature Commun 4:1971, doi:10.1038/ncomms2971
Osken I, Gundogan AS, Tekin E, Eroglu MS, Ozturk T (2013) Fluorene-Dithienothiophene-S, S-dioxide copolymers. Fine-tuning for OLED applications. Macromolecules 46:9202–9210
Puodziukynaite E, Wang L, Schanze KS, Papanikolas JM, Reynolds JR (2014) Poly(fluorene-co-thiophene)-based ionic transition-metal complex polymers for solar energy harvesting and storage applications. Polym Chem 5:2363–2369
Leclerc M (2001) Polyfluorenes: twenty years of progress. J Polym Sci Part A: Polym Chem 39:2867–2873
Zhang T, Wang R, Ren H, Chen Z, Li J (2012) Deep blue light-emitting polymers with fluorinated backbone for enhanced color purity and efficiency. Polymer 53:1529–1534
Montali A, Smith P, Weder C (1998) Poly(p-phenylene ethynylene)-based light-emitting devices. Synth Met 97:123–126
Mishra SP, Palai AK, Kumar A, Srivastava R, Kamalasanan MN, Patri M (2010) Highly air-stable thieno[3,2-b]thiophene–thiophene-thiazolo[5,4-d]thiazole-based polymers for light-emitting diodes. Macromol Chem Phys 211:1890–1899
Poul B, Kap-soo C, Gilles D, Nicolas D, Serge T, David PW, Li W (2011) Photovoltaic cell with benzodithiophene-containing polymer. WO/2011/085004
Mishra SP, Palai AK, Kumar A, Srivastava R, Kamalasanan MN, Patri M (2009) Dithieno[3,2-b:2’,3’-d]pyrrole-alkylthiophene-benzo[c][1, 2, 5]thiadiazole-based highly stable and low band gap polymers for polymer light-emitting diodes. J Polym Sci Part A: Polym Chem 47:6514–6525
Wu Z, Fan B, Li A, Xue F, Ouyang J (2011) Low-band gap copolymers of ethynylfluorene and 3,6-dithiophen-2-yl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione synthesized under microwave irradiation for polymer photovoltaic cells. Org Electron 12:993–1002
Palai AK, Rath SK, Srivastava R, Kamalasanan MN, Patri M (2009) Substituent effect on the optoelectronic properties of poly(p-phenylenevinylene) based conjugated-nonconjugated copolymers. J Appl Polym Sci 112:2988–2998
Palai AK, Mishra SP, Kumar A, Srivastava R, Kamalasanan MN, Patri M (2010) Synthesis and characterization of alternative donor-acceptor arranged poly(arylene ethynylene)s derived from 1,4-diketo-3,6-diphenylpyrrolo-[3,4-c]pyrrole (DPP). Eur Polym J 46:1940–1951
Zhan X, Liu Y, Yu G, Wu X, Zhu D, Sun R, Wang D, Epstein AJ (2001) Synthesis and electroluminescence of poly(aryleneethynylene)s based on fluorene containing hole-transport units. J Mater Chem 11:1606–1611
Mishra SP, Javier AE, Zhang R, Liu J, Belot JA, Osaka I, McCullough RD (2011) Mixed selenium-sulfur fused ring systems as building blocks for novel polymers used in field effect transistors. J Mater Chem 21:1551–1561
Ma X, Azeem EA, Liu X, Cheng Y, Zhu C (2014) Synthesis and tunable chiroptical properties of chiral BODIPY-based D-π-A conjugated polymers. J Mater Chem C 2:1076–1084
Acknowledegments
The authors thank M. N. Kamalasanan and R. Srivastava from NPL, New Delhi for their help in PLED device fabrication and characterization. The authors would also like to thank Professor Seungmoon Pyo, Konkuk University, Seoul, Republic of Korea and Professor Soonmin Jang, Sejong University, Seoul, Republic of Korea for their help in DFT calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palai, A.K., Kumar, A., Mishra, S.P. et al. Fluorene-based conjugated poly(arylene ethynylene)s containing heteroaromatic bicycles: preparation and electro-optical properties. J Mater Sci 49, 7408–7417 (2014). https://doi.org/10.1007/s10853-014-8438-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8438-2