Abstract
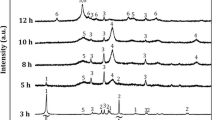
The ball milling-induced reduction of MoS2 by Al has been investigated. Although this is a highly exothermic reaction that, based on its thermodynamic properties, should progress as a self-sustaining process, ignition could not be achieved by ball milling. In order to identify the reason, XRD, particle size distribution, SEM, and DTA measurements were carried out on a series of samples milled for different durations. It was found that the largest composite agglomerates broke up due to the presence of a fine dispersion of MoS2 particles. SEM also revealed that the grains are packed rather loosely within the agglomerates. These results indicate that a self-sustaining process can take place only if large and well-compacted composite particles are present.






Similar content being viewed by others
References
Heinicke G (1984) Tribochemistry. Hanser Publishers, München
Avvakumov EG (1986) Mechanical Methods of the Activation of Chemical Processes. Nauka Publishing House, Novosibirsk
Suryanarayana C (2001) Prog Mater Sci 46:1
McCormick PG (1992) Metall Trans A 23A:1285
Takacs L (1993) NanoStruct Mater 2:241
Takacs L (1996) J Sol State Chem 125:75
Sugamata M, Kaneko J, Higuchi H (1998) Mater Sci Forum 269–272:157
McCormick PG (1995) Mater Trans JIM 36:161
Tsuzuki T, McCormick PG (2004) J Mater Sci 39:5143
Schaffer GB, McCormick PG (1989) Appl Phys Lett 55:45
Takacs L (1992) Mater Lett 13:119
Takacs L (2002) Prog Mater Sci 47:355
Torosyan AR, Martirossyan VG, Karakhanyan SS (1998) Int J Self-Propag High-Temp Synth 7:87
Rodrigues JA et al (1991) J Mater Sci Lett 10:819
Kubaschewski O, Alcock CB, Spencer PJ (1993) Materials Thermochemistry, 6th ed. Pergamon Press, Oxford
Klug HP, Alexander LA (1977) X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd Ed. John Wiley and Sons, New York, p 665
Eckert J, Holzer JC, Krill CE, Johnson WL, (1992) J Mater Res 7:1751
Fecht HJ, Hellstern E, Fu Z, Johnson WL (1990) Metall Trans A 21A:2333
Chakurov Chr, Rusanov V, Koichev J (1987) J Sol State Chem 71:522
Takacs L, Soika V, Baláž P (2001) Sol State Ionics 141–142:641
Acknowledgements
One of the authors (A.R.T.) is expressing his thanks to the Council for International Exchange of Scholars for a Fulbright Scholarship that made this investigation possible. This work was supported in part by DRIF funds from the University of Maryland, Baltimore County. The help of Dr. Robert C. Reno with the SEM investigations and the ball milling experiments carried out by Youssef A. Mahmoud are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
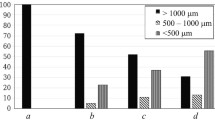
The particle size distribution is characterized in Fig. 3 by plotting the volume fraction density (Q log) as a function of the decadic logarithm of the particle diameter (log x.) From this graph, the volume fraction of particles between diameters x 1 and x 2,
P(x 1 < x< x 2), is obtained as the area under the curve between log x 1 and log x 2. If x 2−x 1 << x 2,
Although the proper variable of the horizontal axis is log x, it is usually labeled in terms of diameters. The logarithm is dimensionless, thus both P and Q log can be expressed in percentages.
The quantity most often used to characterize the particle size distribution is the cumulative distribution F(x), defined as the volume fraction occupied by particles smaller than x. If an atom of the sample is chosen at random, F(x) gives the probability that it belongs to a particle smaller than x. As F is an integral quantity, it may hide some fine details of the particle size distribution. Q log relates to F as follows:
The volume fraction of particles between diameters x 1 and x 2 is
If Δx = x 2−x 1 << x 2,
where Q = dF/dx is the volume fraction density. The dimension of Q is 1/length. Q log differs from Q, because its variable is log(x) rather than x. But Eq. (A.1) and (A.3) provide the same quantity, consequently
From here,
Rights and permissions
About this article
Cite this article
Takacs, L., Baláž, P. & Torosyan, A.R. Ball milling-induced reduction of MoS2 with Al. J Mater Sci 41, 7033–7039 (2006). https://doi.org/10.1007/s10853-006-0950-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0950-6