Abstract
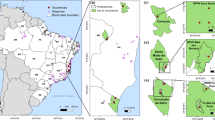
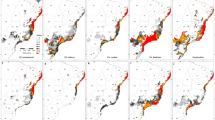
Along with other human impacts, climate change is an important driver of biological changes worldwide and is expected to severely affect species distributions. Although dramatic range shifts and contractions are predicted for many taxa occurring at higher latitudes, including bumble bees, the response of widespread tropical species is less clear due in part to scarcity of reliable occurrence data. Newly mobilized specimen records and improved species distribution models facilitate more robust assessment of future climate effects under various scenarios. Here, we predict both current and future distribution of the orchid bee Eulaema nigrita Lepeletier, 1841 (Apidae: Euglossinae), a large-bodied species widely distributed in the Neotropics whose populations within the Amazon region are believed to be controlled by cleptoparasitic Euglossini bees, such as Exaerete smaragdina Guérin-Menéville, 1844 and Aglae caerulea Lepeletier and Serville, 1825. Under both current and future scenarios of climate change, El. nigrita is expected to persist in deforested areas including those that might suffer desertification. While under current climatic conditions this species is not expected to occur in central Amazonia where the forest is still conserved, its range is expected to increase under future scenarios of climate change, especially in areas corresponding to the arc of deforestation in eastern Amazonia. The increase of human-related disturbances in this biome, as well as changes in the relationship of El. nigrita–Ex. smaragdina and El. nigrita–A. caerulea may explain the potential range increase of El. nigrita under future scenarios of climate change.



Similar content being viewed by others
References
Aguiar WM, Sofia SH, Melo GAR, Gaglianone MC (2015) Changes in orchid bee communities across forest-agroecosystem boundaries in Brazilian Atlantic Forest landscapes. Environ Entomol. doi:10.1093/ee/nvv130
Aiello-Lammens ME, Boria RA, Radosavljevic A et al (2015) spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38:541–545
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, Kappa and the True Skill Statistic (TSS). J Appl Ecol 43:1223–1232
Almeida MC, Côrtes LG, De Marco P Jr (2010) New records and a niche model for the distribution of two Neotropical damselflies: Schistolobos boliviensis and Tuberculobasis inversa (Odonata: Coenagrionidae). Insect Conserv Divers 3:252–256
Araújo MB, Pearson RG (2005) Equilibrium of species’ distributions with climate. Ecography 28:693–695
Arribas P, Abellán P, Velasco J et al (2012) Evaluating drivers of vulnerability to climate change: a guide for insect conservation strategies. Glob Change Biol 18:2135–2146
Ballesteros-Mejia L, Kitching IJ, Jetz W et al (2013) Mapping the biodiversity of tropical insects: species richness and inventory completeness of African sphingid moths. Glob Ecol Biogeogr 22:586–595
Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol Evol 3:327–338
Bartomeus I, Ascher JS, Wagner D et al (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci USA 108:20645–20649
Boria RA, Olson LE, Goodman SM, Anderson RP (2014) Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol Model 275:73–77
Brienen RJW, Phillips OL, Feldpausch TR et al (2015) Long-term decline of the Amazon carbon sink. Nature 519:344–348
Chapin FS, Zavaleta ES, Eviner VT et al (2000) Consequences of changing biodiversity. Nature 405:234–242
De Marco P Jr, Vianna DM (2005) Distribuição do esforço de coleta de Odonata no Brasil: subsídios para escolha de áreas prioritárias para levantamentos faunísticos. Lundiana 6:13–26
De Oliveira G, Rangel TF, Lima-Ribeiro MS et al (2014) Evaluating, partitioning, and mapping the spatial autocorrelation component in ecological niche modeling: a new approach based on environmentally equidistant records. Ecography 37:637–647
De Siqueira MF, Durigan G, De Marco P Jr, Peterson AT (2009) Something from nothing: using landscape similarity and ecological niche modeling to find rare plant species. J Nat Conserv 17:25–32
Diniz-Filho JAF, De Marco P Jr, Hawkins BA (2010) Defying the curse of ignorance: perspectives in insect macroecology and conservation biogeography. Insect Conserv Divers 3:172–179
Domisch S, Araújo MB, Bonada N et al (2013) Modelling distribution in European stream macroinvertebrates under future climates. Glob Change Biol 19:752–762
Dressler RL (1982) Biology of the orchid bees (Euglossini). Annu Rev Ecol Syst 13:373–394
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Elith J, Graham CH, Anderson RP et al (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29:129–151
Evangelista PH, Kumar S, Stohlgren TJ et al (2008) Modelling invasion for a habitat generalist and a specialist plant species. Divers Distrib 14:808–817
Faria LRR, Silveira FA (2011) The orchid bee fauna (Hymenoptera, Apidae) of a core area of the Cerrado, Brazil: the role of riparian forests as corridors for forest-associated bees. Biota Neotrop 11:87–94
Fearnside PM (2001) Soybean cultivation as a threat to the environment in Brazil. Environ Conserv 28:23–38
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49
Foley JA, Defries R, Asner GP et al (2005) Global consequences of land use. Science 309:570–574
Forrest JRK (2015) Plant-pollinator interactions and phenological change: what can we learn about climate impacts from experiments and observations? Oikos 124:4–13
Giannini TC, Acosta AL, Garófalo CA et al (2012) Pollination services at risk: bee habitats will decrease owing to climate change in Brazil. Ecol Model 244:127–131
Giannini TC, Acosta AL, Silva CI et al (2013) Identifying the areas to preserve passion fruit pollination service in Brazilian Tropical Savannas under climate change. Agric Ecosyst Environ 171:39–46
Gonçalves RB, Sydney NV, Oliveira PS, Artmann NO (2014) Bee and wasp responses to a fragmented landscape in southern Brazil. J Insect Conserv 18:1193–1201
Graham CH, Ferrier S, Huettman F et al (2004) New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol Evol 19:497–503
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135:147–186
Hegland SJ, Nielsen A, Lázaro A et al (2009) How does climate warming affect plant–pollinator interactions? Ecol Lett 12:184–195
Henle K, Davies KF, Kleyer M et al (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13:207–251
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hughes I, Hughes L (2000) Biological consequences of global warming: is the signal already apparent? Trends Ecol Evol 15:56–61
Jarnevich CS, Stohlgren TJ, Kumar S et al (2015) Caveats for correlative species distribution modeling. Ecol Inform 29:6–15
Jiménez-Valverde A, Peterson AT, Soberón J et al (2011) Use of niche models in invasive species risk assessments. Biol Invasions 13:2785–2797
Kamino LHY, Stehmann JR, Amaral S et al (2011) Challenges and perspectives for species distribution modelling in the neotropics. Biol Lett 8:324–326
Kramer-Schadt S, Niedballa J, Pilgrim JD et al (2013) The importance of correcting for sampling bias in MaxEnt species distribution models. Divers Distrib 19:1366–1379
Liu CR, Berry PM, Dawson TP, Pearson RG (2005) Selecting thresholds of occurrence in the prediction of species distributions. Ecography 28:385–393
Liu C, White M, Newell G (2011) Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography 34:232–243
Lobo JM, Jiménez-Valverde A, Real R (2008) AUC: a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr 17:145–151
Malhi Y, Roberts JT, Betts RA et al (2008) Climate change, deforestation, and the fate of the Amazon. Science 319:169–172
Martínez-Meyer E, Díaz-Porras D, Peterson A, Yáñez-Arenas C (2013) Ecological niche structure and rangewide abundance patterns of species. Biol Lett 9:20120637. doi:10.1098/rsbl.2012.0637
Martins AC, Silva DP, De Marco P Jr, Melo GAR (2015) Species conservation under future climate change: the case of Bombus bellicosus, a potentially threatened South American bumblebee species. J Insect Conserv 19:33–43
Maués MM, Oliveira PEAM, Kanashiro M (2008) Pollination biology in Jacaranda copaia (Aubl.) D. Don. (Bignoniaceae) at the “Floresta Nacional do Tapajós”, Central Amazon, Brazil. Rev Bras Botânica 31:517–527
McKinney ML (1997) Extinction vulnerability and selection: combining ecological and paleontological views. Annu Rev Ecol Syst 28:495–516
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant–pollinator interactions. Ecol Lett 10:710–717
Memmott J, Carvell C, Pywell RF, Craze PG (2010) The potential impact of global warming on the efficacy of field margins sown for the conservation of bumble-bees. Philos Trans R Soc B Biol Sci 365:2071–2079
Milet-Pinheiro P, Schlindwein C (2005) Do euglossine males (Apidae, Euglossini) leave tropical rainforest to collect fragrances in sugar cane monocultures? Rev Bras Zool 22:853–858
Moline MA, Claustre H, Frazer TK et al (2004) Alteration of the food web along the Antarctic Peninsula in response to a regional warming trend. Glob Change Biol 10:1973–1980
Morato EF (1992) Abelhas euglossini (Hymenoptera, Apidae) coletadas na Amazônia Central. Rev Bras Entomol 36:767–771
Morato EF (1994) Abundância e riqueza de machos de Euglossini (Hymenoptera: Apidae) em mata de terra firme e áreas de derrubada, nas vizinhanças de Manaus (Brasil). Bol do Mus Para Emílio Goeldi 10:95–105
Morato EF (1998) Estudos sobre comunidades de abelhas Euglossini. Anais do III Encontro sobre Abelhas de Ribeirão Preto. Ribeirão Preto, São Paulo, pp 135–143
Moure JS (1967) Descrição de algumas espécies de Euglossine (Hym. Apoidea). Atlas Simp Biota Amaz 55:373–394
Moure JS (2003) As espécies do gênero Eulaema Lepeletier, 1841 (Hymenoptera, Apidae, Euglossinae). Acta Biol Parana 29:1–70
Muscarella R, Galante PJ, Soley-Guardia M et al (2014) ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol Evol 5:1198–1205
Myers JG (1935) Ethological observations on the citrus bee, Trigona silvestriana Vachal, and other neotropical bees (Hym. Apoidea). Trans R Entomol Soc Lond 83:131–142
Nemésio A (2009) Orchid bees (Hymenoptera: Apidae) of the Brazilian Atlantic forest. Zootaxa 2041:1–242
Nemésio A (2011) Euglossa marianae sp. n. (Hymenoptera: Apidae): a new orchid bee from the Brazilian Atlantic Forest and the possible first documented local extinction of a forest-dependent orchid bee. Zootaxa 2892:59–68
Nemésio A (2013a) Are orchid bees at risk? First comparative survey suggests declining populations of forest-dependent species. Braz J Biol 73:367–374
Nemésio A (2013b) The orchid-bee faunas (Hymenoptera: Apidae) of “Parque Nacional do Monte Pascoal”, “Parque Nacional do Descobrimento” and three other Atlantic Forest remnants in southern Bahia, eastern Brazil. Braz J Biol 73:437–446
Nemésio A, Faria-Junior LRR (2004) First assessment of the orchid-bee fauna (Hymenoptera: Apidae) at Parque Estadual do Rio Preto, a cerrado area in southeastern. Lundiana 5:113–117
Nemésio A, Silveira FA (2006a) Deriving ecological relationships from geographical correlations between host and parasitic species: an example with orchid bees. J Biogeogr 33:91–97
Nemésio A, Silveira FA (2006b) Edge effects on the orchid-bee fauna (Hymenoptera: Apidae) at a large remnant of Atlantic Rain Forest in southeastern Brazil. Neotrop Entomol 35:313–323
Nemésio A, Cerântola NCM, Vasconcelos HL et al (2012) Searching for Euglossa cyanochlora Moure, 1996 (Hymenoptera: Apidae), one of the rarest bees in the world. J Insect Conserv 16:745–755
Newbold T (2010) Applications and limitations of museum data for conservation and ecology, with particular attention to species distribution models. Prog Phys Geogr 34:3–22
Nóbrega CC, De Marco P Jr (2011) Unprotecting the rare species: a niche-based gap analysis for odonates in a core Cerrado area. Divers Distrib 17:491–505
Oliveira ML (2008) Catálogo comentado das espécies de abelhas do gênero Eulaema Lepeletier, 1841 (Hymenoptera: Apidae). Lundiana 8:113–136
Oyama MD, Nobre CA (2003) A new climate-vegetation equilibrium state for Tropical South America. Geophys Res Lett 30:2199
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Peruquetti RC, Campos LAO, Coelho CDP et al (1999) Abelhas euglossini (Apidae) de áreas de Mata Atlântica: abundância, riqueza e aspectos biológicos. Rev Bras Zool 16:101–118
Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Rafferty NE, CaraDonna PJ, Bronstein JL (2015) Phenological shifts and the fate of mutualisms. Oikos 124:14–21
Rangel TF, Loyola RD (2012) Labeling ecological niche models. Nat Conserv 10:119–126
Rasmont P, Franzén M, Lecocq T et al (2015) Climatic risk and distribution atlas of European bumblebees, 1st edn. Pensoft Publishers, Sofia
Rebêlo JMM, Silva FS (1999) Distribuição das abelhas Euglossini (Hymenoptera: Apidae) no estado do Maranhão, Brasil. An da Soc Entomológica do Bras 28:389–401
Reddy S, Davalos LM (2003) Geographical sampling bias and its implications for conservation priorities in Africa. J Biogeogr 30:1719–1727
Root TL, Price JT, Hall KR et al (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Schuh RT, Hewson-Smith S, Ascher JS (2010) Specimen databases: a case study in entomology using web-based software. Am Entomol 56:206–216
Schweiger O, Heikkinen RK, Harpke A et al (2012) Increasing range mismatching of interacting species under global change is related to their ecological characteristics. Glob Ecol Biogeogr 21:88–99
Serra BDV, De Marco P Jr, Nóbrega CC, Campos LAO (2012) Modeling potential geographical distribution of the wild nests of Melipona capixaba Moure & Camargo, 1994 (Hymenoptera, Apidae): conserving isolated populations in mountain habitats. Nat Conserv 10:199–206
Silva DP, De Marco PJ (2014) No evidence of habitat loss affecting the orchid bees Eulaema nigrita Lepeletier and Eufriesea auriceps Friese (Apidae: Euglossini) in the Brazilian Cerrado Savanna. Neotrop Entomol 43:509–518
Silva DP, Aguiar AJC, Melo GAR et al (2013) Amazonian species within the Cerrado savanna: new records and potential distribution for Aglae caerulea (Apidae: Euglossini). Apidologie 44:673–683
Silva DP, Gonzalez VH, Melo GAR et al (2014) Seeking the flowers for the bees: integrating biotic interactions into niche models to assess the distribution of the exotic bee species Lithurgus huberi in South America. Ecol Model 273:200–209
Silva DP, Varela S, Nemésio A, De Marco P Jr (2015) Adding biotic interactions into paleodistribution models: a host-cleptoparasite complex of Neotropical orchid bees. PLoS One 10:e0129890. doi:10.1371/journal.pone.0129890
Skov F, Svenning J-C (2004) Potential impact of climatic change on the distribution of forest herbs in Europe. Ecography 27:366–380
Soares-Filho BS, Nepstad DC, Curran LM et al (2006) Modelling conservation in the Amazon basin. Nature 440:520–523
Sousa-Baena MS, Garcia LC, Peterson AT (2014) Knowledge behind conservation status decisions: data basis for “Data Deficient” Brazilian plant species. Biol Conserv 173:80–89
Stockwell DRB, Peterson AT (2002) Effects of sample size on accuracy of species distribution models. Ecol Model 148:1–13
Thomas CD, Cameron A, Green RE et al (2004) Extinction risk from climate change. Nature 427:145–148
Tonhasca A Jr, Blackmer JL, Albuquerque GS (2002) Abundance and diversity of euglossine bees in the fragmented landscape of the Brazilian Atlantic forest. Biotropica 34:416–422
Tôrres NM, De Marco P Jr, Santos T et al (2012) Can species distribution modelling provide estimates of population densities? A case study with jaguars in the Neotropics. Divers Distrib 18:615–627
Tougou D, Musolin DL, Fujisaki K (2009) Some like it hot! Rapid climate change promotes changes in distribution ranges of Nezara viridula and Nezara antennata in Japan. Entomol Exp Appl 130:249–258
Tsoar A, Allouche O, Steinitz O et al (2007) A comparative evaluation of presence-only methods for modelling species distribution. Divers Distrib 13:397–405
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11:1351–1363
Ureña-Aranda CA, Rojas-Soto O, Martínez-Meyer E et al (2015) Using range-wide abundance modeling to identify key conservation areas for the micro-endemic Bolson Tortoise (Gopherus flavomarginatus). PLoS One 10:e0131452. doi:10.1371/journal.pone.0131452
VanDerWal J, Shoo LP, Graham C, William SE (2009) Selecting pseudo-absence data for presence-only distribution modeling: how far should you stray from what you know? Ecol Model 220:589–594
Varela S, Anderson RP, García-Valdés R, Fernández-González F (2014) Environmental filters reduce the effects of sampling bias and improve predictions of ecological niche models. Ecography 37:1084–1091
Veloz SD (2009) Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J Biogeogr 36:2290–2299
Whittaker RJ, Araújo MB, Jepson P et al (2005) Conservation biogeography: assessment and prospect. Divers Distrib 11:3–23
Williams H, Mark W (1983) Orchid floral fragrances and male euglossine bees: methods and advances in the last sesquidecade. Biol Bull 164:355–395
Zucchi R, Sakagami SF, Camargo JMF (1969) Biological observations on a neotropical parasocial bee, Eulaema nigrita, with a review on the biology of Euglossinae (Hymenoptera, Apidae). J Fac Sci Hokkaido Univ Ser VI Zool 17:271–380
Acknowledgments
The authors are in debt to Dr. André Nemésio, who kindly revised our occurrence dataset and suggested the inclusion and removal of doubtful records from our analyses and to two anonymous reviewers who provided important suggestions to a previous version of this manuscript. DPS received a doctorate scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (147204/2010-0). PDMJ have been continuously supported by CNPq grants. DPS and PDMJ also thank CNPq (477639/2010-0), Fundação “O Boticário” de Proteção à Natureza (0880/2010-2), and Whitley Wildlife Conservation Trust for the resources which allowed them to execute field surveys in the Cerrado from the state of Goiás. They are also grateful to Dário P. Silva Jr., Mírian Cristina de Almeida, and Fábio Martins Vilar de Carvalho and several others for their help during the field campaigns. Data acquisition on Euglossini records at the American Museum of Natural History by JSA with help from HH Go, A Pfister, M Tuell, and ES Wyman was supported by RG Goelet and NSF-DBI #0956388 (P.I. JS Ascher, co-P.I.s JG Rozen Jr., and D Yanega).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silva, D.P., Macêdo, A.C.B.A., Ascher, J.S. et al. Range increase of a Neotropical orchid bee under future scenarios of climate change. J Insect Conserv 19, 901–910 (2015). https://doi.org/10.1007/s10841-015-9807-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-015-9807-0