Abstract
Iron (Fe) is an important nutrient for phytoplankton. The low solubility of Fe in oxic waters can be a growth-limiting factor for phytoplankton. Synthetic aminopolycarboxylates (APCs) such as ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetic acid (DTPA) are widely used as Fe complexing agents for microalgae culture. However, the presence of these non-ready biodegradable APCS in aquatic systems may have serious environmental consequences. In the present study, the effects of biodegradable chelating ligands (hydroxyiminodisuccinic acid (HIDS), methylglycinediacetic acid (MGDA), and iminodisuccinate (IDS)) on Fe uptake in and growth of three coastal microalgae (Heterosigma akashiwo, Prymnesium parvum, and Skeletonema marinoi-dohrnii complex) were investigated, and the results were compared with those of non-ready biodegradable APCs (EDTA, ethylenediamine tetra-methylene phosphonic acid (EDTMP), and DTPA). The biodegradable chelating ligands did not have significant growth inhibition effect on the phytoplankton. Although the growth of the algae (except S. marinoi-dohrnii complex) was not affected substantially by 1.5 and 7.5 μM of DTPA, growth inhibition occurred by 7.5 μM of EDTMP and 150 μM of EDTA, DTPA, and EDTMP. The effect of chelating ligands on microalgal growth was likely to be associated with the intracellular Fe uptake influenced by the chelating ligands. On average, intracellular Fe concentrations for biodegradable chelating ligands were substantially higher than those for non-ready biodegradable APCs. Except H. akashiwo, the ratio of intra/extracellular Fe concentrations was highest for MGDA followed by IDS and HIDS. The results indicate that biodegradable chelating ligands are more efficient than non-ready biodegradable APCs in intracellular Fe uptake and algal growth.
Similar content being viewed by others
Introduction
Microalgae are currently cultivated commercially for human nutritional products and biofuels around the world in several dozen small- to medium-scale production systems, producing a few tons to a several hundreds of tons of biomass annually (Benemann 2008). Total world production of dry algal biomass is estimated at about 10,000 t per year, of which about half is produced in mainland China, with most of the rest in Japan, Taiwan, the USA, Australia, India and a few small producers in some other countries (Benemann 2008). Due to high lipid content (20–50% and up to 80% in some cases), microalgae are also considered a promising feedstock for biofuel and next-generation renewable energy source (Chisti 2007; Benvenuti et al. 2016). Algal biofuel production has been extensively researched for over three decades, and a number of challenges have been identified in commercial microalgae production (Lin et al. 2011). These include lipid content, optimum growth condition, and productivity of the microalgae.
Iron (Fe) is an important nutrient for phytoplankton metabolism where it is required for photosynthetic and respiratory electron transport, nitrate reduction, chlorophyll synthesis, and detoxification of reactive oxygen species (Sunda and Huntsman 1995; Marchetti & Maldonado 2016). Iron is the fourth most abundant element in the earth’s crust (Anderson and Morel 1982); however, the oxidized form of Fe, Fe(III), is sparingly soluble in oxygenated waters and, therefore, occurs at extremely low concentration (Wells et al. 1995). This suggests that phytoplankton may be Fe-limited in some aquatic systems that may affect the growth of phytoplankton. Studies have shown that limited Fe availability impairs phytoplankton growth by as much as 40% of the open oceans (Moore et al. 2001), notably in the high-nutrient low-chlorophyll (HNLC) and oligotrophic waters near the Equator and further south (Beardall et al. 2001; Blain et al. 2007).
The economic feasibility of algal culture for nutritional products and/or biofuel production greatly depends on the high biomass productivity and appreciable lipid yields that are significantly affected by Fe nutrition (Liu et al. 2008). Synthetic aminopolycarboxylates (APCs), such as ethylenediaminetetraacetic acid (EDTA), were introduced during the 1950s as a metal ion buffer in seawater media for algal culture (Kean et al. 2015) and since then have been commonly used in order to mimic low Fe concentrations in algal culture (Gerringa et al. 2000). Most of the research has been performed in laboratory cultures using phytoplankton; EDTA had been added in either a synthetic medium or filtered natural seawater in order to buffer the metal chemistry (Anderson and Morel 1982; Sunda et al. 1991; Sunda and Huntsman 1995; Gerringa et al. 2000). In the past decades, there have been many experiments investigated the response of marine phytoplankton productivity to Fe additions in natural sea water without or with low addition of EDTA (Martin et al. 1991; Coale et al. 1996a,b; Boyd et al. 1998) and in laboratory media (Gerringa et al. 2000). Anderson and Morel (1982) investigated Fe uptake in coastal diatom Thalassiosira weissflogii under well-defined chemical conditions imposed by a large excess of the APCs (EDTA, cyclohexanediaminetetraacetic acid (CDTA), diethylenetriaminepentaacetic acid (DTPA), and nitrilotriacetic acid (NTA)). Results showed that the Fe uptake rate was a function of the activity of the Fe3+ ion in the absence of light. However, in the absence of the APCs, Fe uptake rates were dependent on the total Fe concentrations for both Fe(II) and Fe(III) additions to the medium.
EDTA has a fairly high affinity for transition metals and was introduced as a trace metal buffering reagent for algal cultures by Hutner et al. (1950) and Myers et al. (1951). One of the important purposes of the use of EDTA in algal culture medium was to obtain a constant and controlled supply of Fe (Gerringa et al. 2000). However, the fact is that EDTA and other synthetic non-ready biodegradable APCs lead to considerable amounts in the aquatic environment if they are used for algal culture (Pinto et al. 2014). It is true that industrial settings in which environmental release can be controlled or adequately treated may, however, benefit from a chelate that is active for longer. In the past decades, discussion on the environmental consequences of non-ready biodegradable APCs has been raised. The biodegradation of synthetic APCs such as EDTA and DTPA is very limited, and they can only be biodegraded in very particular conditions, with specific isolated bacterial strains (Bucheli-Witschel and Egli 2001; Nörtemann 2005). For example, in a closed bottle test, Bucheli-Witschel and Egli (2001) showed that EDTA can be completely degraded at pH 8. They also showed that the more stable forms of EDTA (such of Fe-EDTA) have to dissociate before degrading and, hence, are recalcitrant to microbial attack under many conditions. With this finding, still these compounds do not follow the Organization for Economic Cooperation and Development (OECD) criteria for biodegradability (Knepper 2003). Therefore, the replacement of these compounds by biodegradable alternatives has been the object of studies in the last three decades.
Recently, we have extensively studied the influence of non-ready biodegradable APCs and biodegradable chelating ligands on Fe mobility and bioavailability in growth medium and its uptake in plants (Rahman et al. 2009, 2011; Hasegawa et al. 2010, 2011, 2012). From these studies, we concluded that the concentration of chelating ligands and the stability constant of Fe-L complex (KFe-L) in the medium are important factors for Fe bioavailability to and uptake in plants. Iron exists predominantly as soluble [Fe(OH)2+, Fe(OH)2+, Fe-L complexes] and apparently soluble (colloidal) forms at moderate concentration of the chelants, which is favorable for Fe bioavailability and uptake (Hasegawa et al. 2012). Furthermore, the biodegradable ligands (e.g., hydroxyiminodisuccinic acid (HIDS)) are more efficient than the non-ready biodegradable APCs (e.g., EDTA) for Fe uptake in plants (Rahman et al. 2009; Hasegawa et al. 2012). However, the effects of biodegradable chelating ligands on Fe uptake in and growth of phytoplankton have not been demonstrated.
In the present study, the effects of synthetic chelating ligands on Fe uptake (using a radioactive tracer 55Fe) and growth (using spectrophotometer) of three coastal phytoplankters (Heterosigma akashiwo, Prymnesium parvum, and Skeletonema marinoi-dohrnii complex) were investigated by growing them in artificial seawater at different concentrations of biodegradable chelating ligands (HIDS, methylglycinediacetic acid (MGDA), and iminodisuccinate (IDS)) and the results were compared with those of non-ready biodegradable APCs (EDTA, ethylenediamine tetra-methylene phosphonic acid (EDTMP), and DTPA). We examined differential effects of chemical properties and ligands’ concentrations in the culture medium on the growth of phytoplankton. The objective of the study was to identify biodegradable chelating ligands that can be used as alternatives to the environmentally persistent APCs for phytoplankton cultivation.
Materials and methods
Reagents
Ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA; > 98.0%, Dojindo, Japan), diethylenetriamine-N,N,N′,N″,N″-tetraacetic acid (DTPA; > 99.8%, Dojindo, Japan), ethylenediamine-N,N,N′,N′-methylene(phosphonic acid) (EDTMP; 95.0%, Dojindo, Japan), hydroxyiminodisuccinic acid (HIDS; purity 90.19%, Nippon Shokubai, Japan), iminodisuccinate (IDS; 34%, Rankusasu, Japan), and methylglycinediacetic acid (MGDA; 82.0–84.0%, BASF, USA) were used as APCs in the present study. Iron chloride hexahydrate (FeCl3·6H2O; special grade, Wako, Japan) was used for the preparation of nutrient solution. The standard solution of Fe was prepared by dissolving FeCl3·6H2O (Wako, Japan), and radioactive isotope 55Fe was prepared by dissolving 55FeCl3 (740 MBq, Daiichi Pure Chemicals, Japan) in 1 M HCl. They were diluted to the desired concentrations with E-pure water (EPW, E-pure system, Barnstead, USA).
Phytoplankton
Three coastal phytoplankton (Heterosigma akashiwo 893, Prymnesium parvum, and Skeletonema marinoi-dohrnii complex NIES-16) were used in the present study. The strains of H. akashiwo and P. parvum were gently provided by Prof. I. Imai, Hokkaido University, Japan.
Heterosigma akashiwo is a microscopic alga of the class Raphidophyceae. Red tides of this species have been reported in coastal waters of Japan (Yamochi 1989), Korea (Park 1989), Singapore and New Zealand (Chang et al. 1990; Chang et al. 1993), and Canada (Taylor and Haigh 1993).
Prymnesium parvum is an ichthyotoxic phytoplankton (Haptophyta) growing in geographically widespread areas of the low-nutrient open oceans including temperate waters of both the northern and southern hemispheres (Fistarol et al. 2003), vast areas of temperate and tropical open seas (Edvardsen and Imai 2006), the Baltic Sea (Edler et al. 1984), and many coastal areas (Edvardsen and Imai 2006; Carvalho and Granéli 2010). Haptophyte algae are important primary producers in aquatic environments, and they are probably the main primary carbon source for petroleum products (Andersen 2004). It is tolerant of large variations in temperature and salinity (Lundholm and Moestrup 2006; Baker et al. 2007, 2009). Because of its widespread distribution in low-nutrient open oceans, P. parvum has been used in many oceanographic studies during the last decade (Fistarol et al. 2003; La Claire 2006; Baker et al. 2007; Maki et al. 2008; Carvalho and Granéli 2010; Roelke et al. 2010; Susan et al. 2010; Rahman et al. 2014). Skeletonema marinoi-dohrnii inhabits bottom sediments in the form that allow survival under cold, dark conditions (Itakura et al. 1992, 1997; Shikata et al. 2008). As a cosmopolitan alga, S. marinoi-dohrnii has been used as a model organism in numerous studies.
Pre-culture and maintenance
Axenic strains of coastal phytoplankton were maintained for 2 weeks in 30-mL polycarbonate bottles (Nalge, USA) with artificial seawater containing modified f/2 nutrient solution (Table 1) in which 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethane sulfonic acid (HEPES; Nacalai Tesque, Japan) was used as a buffer reagent (Watanabe et al. 2000). Axenicity was assessed and monitored by 4′,6-diamidino-2-phenylindole (DAPI) test. Artificial seawater was prepared according to the method of Lyman and Fleming (1940). Deionized water used for the preparation of modified f/2 nutrient solution was purified using E-pure (Barnstead, Thermo Fisher Scientific, Inc., USA) system.
Vessels, bottles, tips, and micropipettes used in the test were sterilized by autoclaving at 121 °C for 30 min. Modified f/2 nutrient solution and FeCl3 (10 μM) solution (in 1 M HCl) were sterilized separately to avoid Fe contamination. The FeCl3 solution was then mixed with the modified f/2 nutrient solution. After sterilization, the materials were placed in a clean bench and kept under UV irradiation for 20 min. Phytoplankton were grown in modified f/2 nutrient solution in artificial seawater in 30-mL polycarbonate bottles (Nalge, USA), and the experiment was conducted in a temperature- and light-controlled incubator (Koitotron 3HN-35MLA, Koito Industries, Ltd. Japan). The culture conditions in the incubator were: 12/12 h light/dark photoperiod at a light intensity of 180 μmol photons m−2 s−1 at 20 °C.
Growth experiment under different chelating ligand concentrations
The axenic algal strains were pre-cultured for 2 weeks in 30-mL polycarbonate bottles with 30 mL of artificial seawater containing modified f/2 nutrient solution containing 0.01 μM Fe. Ten milliliter of pre-culture solution of the phytoplankton at logarithmic growth phase was inoculated in 1.8 L of modified f/2 nutrient solution with 1.5 μM EDTA and 1.5 μM FeCl3 in 2-L polycarbonate bottles under laminar air flow. After 24 h, 30-mL aliquots of the cultures were pipetted into 30-mL acid-washed polycarbonate bottles. The phytoplankton were cultured in the same manner as maintenance cultures, and the additional 1.5, 7.5, and 150 μM of biodegradable (HIDS, MGDA, and IDS, respectively) and non-ready biodegradable (EDTA, DTPA, and EDTPO, respectively) ligands were added by adding appropriate amount of 3 mM solutions of the respective ligands at day 4. The pH of the cultures was adjusted to 8.0 using 0.1 M HCl and NaOH. Then, the phytoplankton were grown for 2 weeks.
Iron uptake experiment with different chelating ligands
The axenic strains of the phytoplankton were pre-cultured in 30 mL of artificial seawater containing f/2 nutrient solution containing 0.01 μM Fe without chelating ligands. From this pre-culture, 2.2 mL solution of algal culture (about 2 × 103 cells mL−1) was inoculated in 400 mL modified f/2 nutrient solution without Fe and chelating ligand until the cells were at a logarithmic phase of growth.
Then, 30-mL aliquots of the algal pre-culture of logarithmic growth phase were pipetted into 30-mL acid-washed polycarbonate bottles and were adjusted to 15 μM chelating ligand and 1.5 μM FeCl3 including 1.25 MBq L−1 of 55Fe. The pH of the culture solution was adjusted to 8.0 using 0.1 M HCl and NaOH. The algal cultures were grown for 48 h in the same manner as maintenance culture.
Extra- and intracellular Fe concentrations were determined according to the method of Hudson and Morel (1989). Briefly, 3 mL of aliquots were collected from each bottle and filtered through 3-μm pore size filters (Nuclepore, Sigma-Aldrich) in a clean booth. For intracellular Fe measurement, the filter was successively rinsed with 5 mL of the artificial seawater, 5 mL of 0.047 M Ti(III)/citrate/EDTA solution, and 5 mL of the artificial seawater on a filter holder. For total Fe measurement (corresponding to intra plus extracellular Fe), the filter was rinsed only with 5 mL of the artificial seawater. Then, the filters, on which 55Fe retained as a tracer, were directly added to 5 mL of the liquid scintillation solutions (2-(4-tert-butylphenyl)-5-(4-biphenylyl)-1,3,4-oxadiazole 3.03 g per 500 mL toluene) in 20-mL vials. A radiochemical activity of 55Fe was measured using the tritium mode of the liquid scintillation counter. The Fe concentration was calculated by the Fe/55Fe ratio in the samples (Okumura et al. 2013).
Instrumentations
The growth of phytoplankton was measured spectrophotometrically at 540 nm and correlated with an established cell density-to-absorbance ratio to estimate cell number. Radiochemical measurements for 55Fe tracer were carried out on a liquid scintillation counter (LSC-6101, Aloka, Japan). An inductively coupled plasma atomic emission spectrometer (ICP-AES; Optima 3300XL, PerkinElmer, USA) equipped with an ultrasonic nebulizer (U-5000AT, Cetac, USA) was used for the determination of total Fe concentration in standard solution.
Statistics
The growth and intra and extracellular Fe concentration data were subjected to analysis of variance (ANOVA) to determine significant differences using SPSS (v17 for windows). A p value of ≤ 0.05 was considered statistically significant.
Results
Effect of EDTA analogues on Fe uptake
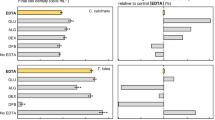
For P. parvum, intracellular Fe concentration in control treatment was 1.74 ± 0.15 nmol cell−1, while its concentration increased slightly in EDTA (2.30 ± 0.72 nmol cell−1) and DTPA (2.10 ± 0.22 nmol cell−1) treatments. A decrease in intracellular Fe uptake was observed for EDTMP treatment. Extracellular Fe concentrations did not differ between the control and the chelating ligand treatments for this microalga (Fig. 1). Extracellular Fe uptake was also not affected by EDTA analogues for S. marinoi-dohrnii. For H. akashiwo, intracellular Fe concentration was found to be significantly higher (p < 0.05) in EDTA and DTPA treatments than in control and EDTMP treatments (Fig. 1). An opposite trend of intracellular Fe uptake was observed for S. marinoi-dohrnii. Although extracellular Fe concentration in EDTA treatment for H. akashiwo was higher than the control and other EDTA analogues, they did not differ significantly (p > 0.05).
Intra- and extracellular Fe concentrations of marine microalgae influenced by non-ready biodegradable chelating ligands (EDTA, DTPA, and EDTMP). Iron and APC concentrations in the growth medium were 1.5 and 15 μM, respectively. Iron concentration in the phytoplankton cells was determined after 48 h of incubation. Error bars are the standard deviations of three replicated samples
Effect of biodegradable chelating ligands on Fe uptake
Biodegradable chelating ligands have significant influence on intra- and extracellular Fe uptake in marine phytoplankton. For P. parvum, intracellular Fe concentration was highest in MGDA treatment compared to control, HIDS, and IDS treatments. The lowest extracellular Fe concentration was in MGDA and HIDS treatments for both P. parvum and S. marinoi-dohrnii (Fig. 2). For S. marinoi-dohrnii, intracellular Fe concentration was highest in IDS treatment compared to control, HIDS, and MGDA. The biodegradable chelating ligands did not have significant effect on intracellular Fe uptake for H. akashiwo. However, for this microalga, extracellular Fe concentration was highest in IDS treatment followed by MGDA treatment (Fig. 2). The results indicate that the effect of biodegradable chelating ligands on intracellular Fe uptake differ with microalgal strains, and MGDA and IDS increased intracellular Fe uptake significantly compared to HIDS.
Intra- and extracellular Fe concentrations of marine microalgae influenced by biodegradable (HIDS, MGDA, and IDS) and non-ready biodegradable (EDTA) chelating ligands. Iron and APC concentrations in the growth medium were 1.5 and 15 μM, respectively. Iron concentration in the phytoplankton cells was determined after 48 h of incubation. Error bars are the standard deviations of three replicated samples
Compared to non-ready biodegradable chelating APCs (EDTA and its analogues), the ratio of intra/extracellular Fe concentrations where substantially higher in biodegradable chelating ligands (Fig. 3). Among biodegradable chelating ligands, the ratio of intra/extracellular Fe concentration was highest in MGDA treatment for P. parvum, while the ratio was almost similar in MGDA, IDS, and HIDS treatments for H. akashiwo (Fig. 3).
Effect of EDTA analogues on microalgal growth
Non-ready biodegradable EDTA and its analogues have significant effect on microalgal growth. EDTMP inhibit the growth of phytoplankton substantially, and the inhibitory effect microalgal growth appeared to be stronger at higher concentrations of the APCs (Fig. 4). The growth of P. parvum was not affected by EDTA; however, its growth was reduced substantially by 7.5 μM of EDTMP and 150 μM of DTPA and EDTMP. The growth of H. akashiwo was not affected by EDTA too, but its growth was completely suppressed by 7.5 and 150 μM EDTMP and 150 μM DTPA (Fig. 4). All the three APCs suppressed the growth of S. marinoi-dohrnii at 1.5, 7.5, and 150 μM concentrations, and a complete stop of the growth of this microalga was observed at 7.5 and 150 μM EDTMP (Fig. 4). Irrespective of the microalgal strain, the overall trend of growth inhibition of phytoplankton by the EDTA analogues was EDTMP > DTPA > EDTA.
Influence of non-ready biodegradable chelating ligands (EDTA, DTPA, and EDTMP) on the growth of marine microalgae. Initially, the microalgae were grown with 1.5 μM Fe and 1.5 μM of EDTA in artificial seawater containing f/2 culture solution. After 4 days, additional 1.5, 7.5, and 150 μM of EDTA, DTPA, and EDTPO were added to the growth medium
Effect of biodegradable chelating ligands on microalgal growth
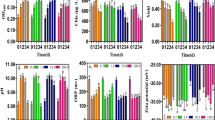
Irrespective of the ligands’ concentrations in the culture medium, the growth of P. parvum and H. akashiwo increased gradually until day 14 (Fig. 5). Both concentrations and chemical properties of the biodegradable chelating ligands influenced the growth of S. marinoi-dohrnii substantially. For 1.5 and 7.5 μM HIDS and IDS treatments, the growth of S. marinoi-dohrnii increased initially (until day 8), and then their growth stopped. However, 1.5 and 7.5 μM MGDA treatments did not have inhibitory effect on the growth of S. marinoi-dohrnii. Initial increase in S. marinoi-dohrnii growth was observed at 150 μM HIDS, IDS, and MGDA treatments, but growth inhibition occurred by the chelating ligands for culturing them for longer time.
Influence of biodegradable chelating ligands (APCs; HIDS, MGDA, and IDS) on the growth of costal phytoplankton. Initially, the microalgae were grown with 1.5 μM Fe and 1.5 μM of EDTA in artificial seawater containing f/2 culture solution. After 4 days, additional 1.5, 7.5, and 150 μM of HIDS, MGDA, and IDS were added to the growth medium
Discussion
Effects of chelating ligands on Fe uptake in and growth of phytoplankton
Regardless of the microalgal species, growth inhibition occurred at high concentrations of synthetic non-ready biodegradable APCs. In addition, the growth inhibition effect of the synthetic APCs appeared to vary depending on the microalgal species (Fig. 4). With the addition of non-ready biodegradable APCs to the culture medium, the growth of phytoplankton increased initially for few days, and then their growth stopped (Fig. 4). Except H. akashiwo, this growth inhibitory effect of synthetic APCs on the phytoplankton was associated with their influence on Fe uptake in microalgal cells (Fig. 1). However, the essential micronutrients such as Se, Co, Zn, Mn, Cu, and Mo remained at > 50% of initial concentrations in the test medium (data not shown). Generally, the uptake of colloidal and soluble iron, i.e., dissolved iron, is the norm of microalgae (Naito et al. 2008). However, it is well established that Fe solubility in oxic waters is too low that phytoplankton can be Fe-limited under such aquatic systems (Anderson and Morel 1982). Synthetic APCs are commonly used in microalgal cultures in order to mimic low Fe concentrations (Gerringa et al. 2000). In previous studies, we reported that the concentrations of chelating ligands and stability constant of Fe-L complex (KFe-L) have significant effect on Fe3+ solubility, bioavailability, and uptake by higher plants (Hasegawa et al. 2011, 2012). The present study also showed that chemical properties and concentrations of synthetic APCs have significant influence on Fe uptake in and growth of phytoplankton.
Growth inhibition of the phytoplankton at high APC concentrations (Fig. 4) and inhibition of intracellular Fe uptake by the phytoplankton for the addition of 15 μM APCs to the culture medium (Fig. 1) indicate that high concentrations of APCs may increase the solubility of Fe3+ in the culture medium, but it does not favor Fe uptake in and growth of the phytoplankton. Thus, being a good Fe fertilizer for phytoplankton (Gerringa et al. 2000), the concentration of APCs in the growth medium can be an important controlling factor for Fe bioavailability and uptake in phytoplankton. Ferric ions and their complexes, which have low solubility in water, are extensively buffered by chelation (Morel and Hering 1993) and increase their dissolved concentrations. In this study, the increased growth of phytoplankton at lower APC concentrations (1.5 and 7.5 μM) than of higher concentration (150 μM) is likely due to the fact that the organisms take up Fe as ferric chelants. However, most of the Fe 3+ in the culture medium formed may soluble Fe-L complexes at high APC concentration (Hasegawa et al. 2012), which may not be bioavailable for the phytoplankton. In addition, the bioavailability of Fe3+ at high APC concentrations may be decreased because of the lopsided complex formation equilibrium reaction (CFER) between Fe3+ and APCs (Hasegawa et al. 2012) that results in the decrease of Fe uptake (Fig. 1) and growth of the phytoplankton (Fig. 4). The suppresses of marine phytoplankton growth, > 50%, at higher concentration of EDTA, 100 μM inhibit cell division, reported from the Northeast Subarctic Pacific (Muggli and Harrison 1996). Iron uptake by marine microalgae decreases with the increase of affinity constant of Fe-ligand complex and ligand-Fe ratios (Sutak et al. 2012). Hutchins et al. (1999) reported that the addition of strong chelating ligand (desferrioxamine B) resulted in artificial Fe limitation to the phytoplankton community in high Fe areas of upwelling region, which is in agreement of the results of our present study.
Although Fe uptake in and growth of phytoplankton were found to be inhibited by non-ready biodegradable APCs, the opposite was observed for biodegradable chelating ligands. The results of the present study showed that, except S. marinoi-dohrnii, the growth of the phytoplankton increased at all concentrations of HIDS, IDS, and MGDA (Fig. 5), and intracellular Fe uptake was substantially higher for the biodegradable chelating ligands than for the non-ready biodegradable APCs (Fig. 3). The extended growth of microalgae after addition of biodegradable chelants in the growth medium was also supported by growing them initially for 4 days with strong chelant, e.g., EDTA. These differential effects of non-ready biodegradable APCs and biodegradable chelating ligands on Fe uptake in and growth of phytoplankton can be explained by their chemical properties and nature of complexation with Fe3+ in aqueous solution (e.g., protonation, stability constant). The protonation and stability constant of synthetic APCs with Fe3+ are higher than those of biodegradable chelating ligands (Pinto et al. 2014). For example, at 25 °C, the stability constant of EDTA with Fe3+ (Fe-EDTA) is 25.1 (Martell and Smith 2004), which is substantially higher than that of biodegradable HIDS (15.0) (Begum et al. 2012), IDS (13.9) (Hyvönen et al. 2003), and MGDA (16.5) (Pinto et al. 2014). In the present study, Fe is likely exists in the culture medium predominantly as inorganic [Fe3+, Fe(OH)2+, Fe(OH)2+, etc.] and colloidal (apparently soluble) forms at 1.5, 7.5, and 150 μM of the biodegradable chelating ligands due to their low stability constants. On the other hand, most of the Fe in the culture medium is likely form Fe-L with EDTA and its analogues (DTPA and EDTMP) at higher (150 μM in this instant) concentration due to their high stability contestants (Hasegawa et al. 2012). As inorganic and colloidal forms of Fe is more bioavailable than Fe-L complex, Fe uptake in and growth of the phytoplankton were higher for biodegradable chelating ligands than those of non-ready biodegradable APCs.
Iron uptake model of phytoplankton under conditions induced by chelating ligands
Composition of Fe(III) species in oxygenated seawater is controlled by complexation and precipitation reaction. Before phytoplankton take up Fe into the cells, Fe(III) species in the medium are supplied on the cell surface, transformed into suitable forms, and then transported across the cell membranes (Marchetti & Maldonado 2016). From the view point of Fe transport systems in phytoplankton, many studies have been made on the two common strategies for Fe acquisitions: biologically mediated reduction of Fe(III) compounds by reductases located on the cell surface followed by Fe(II) uptake (Anderson and Morel 1982; Hudson and Morel 1990) and excretion of iron transporters such as siderophores and citrate that form specific Fe compounds and are subsequently taken up (Trick et al. 1983; Sandy and Butler 2009). There is no firm information to date the latter transport system for eukaryotic phytoplankton. The chelated Fe(III) are not taken up directly across the cell membranes. Berne and Levy (1998) reported that synthetic chelating ligands are too large and polar to move through the plasmalemma lipid bilayer.
Effects of the chelating ligands on the Fe speciation in the medium and the Fe uptake system of eukaryotic phytoplankton are presented in Fig. 6. The chelating ligands, L, complex with inorganic Fe(III) though chelation and produce a soluble species, Fe(III)L. The concentrations and stability constants of the chelating ligands decide the composition of Fe species in the culture medium. The solubility of inorganic Fe(III) in oxygenated seawater without chelating ligands is extremely low (Wells et al. 1995), which limits the supplied amount of Fe to phytoplankton. Addition of chelating ligands have been used to enhance the Fe(III) solubility and to increase the Fe supply to phytoplankton (Morel and Hering 1993).
In this study, the biodegradable chelating ligands, IDS, HIDS, and MGDA, showed some positive effect on the growth of phytoplankton (Fig. 5). Compared to the control and EDTA, the ratio of intra/extracellular Fe concentrations for biodegradable chelating ligands is substantially higher than that for the non-ready biodegradable chelating APCs (Fig. 3). According to the Fe-binding abilities of the biodegradable ligands (log KFeL = 13.9, 15.0, and 16.5 for IDS, HIDS, and MGDA, respectively), the dissolved Fe in the medium is not only Fe(III)L but also unchelated Fe(III), Fe(III)′, at chemical equilibrium. Dissolved Fe(III)′ is partially found in the colloidal size fraction. Dissolved Fe(III) species in the presence of the biodegradable ligands could be precipitated on the algal cells as colloidal form of Fe(III)′. Recent studies have demonstrated that weak ligands, such as saccharides and other exopolymeric substances, could facilitate access of phytoplankton to iron by more readily disassociating and releasing Fe(III)′ to the cell surface (Hassler et al. 2011, 2015). The biodegradable chelating ligands have characteristic properties of moderate complexing ability and microbial degradability, similar to the exopolymeric substances. Another pathway is direct adsorption of dissolved Fe(III)L on the cell surface. Partial framework of amino acid which the biodegradable chelating ligands compose of might facilitate Fe(III)L adsorption on the secretion-rich cell surface. The composition of the extracellular Fe seems to be different by inorganic and organic structures of the cell surface because the contents of the extracellular Fe are influenced by phytoplankton species and chemical forms of chelating ligands (Figs. 1 and 2).
The feature of biodegradable chelating ligands for enhancement of Fe supply is to keep stable growth of phytoplankton (Fig. 5). The Fe pool on the cell surface is potentially important for the uptake of iron, although microalgal growth is supported by the solubility of organically complexed Fe(III) in the media. The uptake pathway (the way of accumulation) on the cells is more entropically favored than the direct uptake of dissolved Fe species into the cells. The microalgae can utilize the Fe(III)L and Fe(III)′ as the Fe source through the reduction to Fe(II) at the cell surface (Shaked et al. 2005). Several mechanisms have been proposed by which phytoplankton could access Fe(III)L and Fe(III)′, including reduction of the Fe(III) by membrane-bound enzymes or by light (Shaked and Lis 2012). Laboratory experiments have shown that the growth rate of phytoplankton is closely correlated with intracellular Fe concentrations (Sunda and Huntsman 1995).
On the other hand, a large excess of the stronger binding ligands, EDTA, EDTMP, and DTPA, inhibited the microalgal growth (Fig. 4), suggesting that complexation of Fe(III)L in the medium and Fe uptake of phytoplankton are competitive. The growth of phytoplankton was inhibited by the deficient Fe(III)′ due to the addition of higher level of chelating ligands (Shaked and Lis 2012). In another way, the adding of strong chelating ligand may increase iron transport to a cell possibly by transporting ligand-bound Fe through cell membrane, exchanging Fe between ligand and a binding group at cell surface, and supplying enhance Fe at cell surface (Jackson and Morgan 1978). However, the composition of truly dissolved and colloidal Fe(III)′ fractions depends on the concentration of the ligands and stability constant of Fe(III)L. Early works demonstrated that Fe uptake of phytoplankton was proportional to the sum of dissolved inorganic Fe(III) species corresponding to the unchelated Fe(III) (Anderson and Morel 1982; Hudson and Morel 1990; Sunda and Huntsman 1995).
Conclusion
Intracellular Fe concentration for biodegradable chelating ligands was substantially higher than that for non-ready biodegradable EDTA analogues. This reflects the growth reduction of phytoplankton by EDTA analogues compared to biodegradable chelating ligands. The impact of chelating ligands on algal growth is likely to be dependent on the uptake pathway, which depended on types and concentrations of chelating ligands and speciation of iron in the medium. A large excess of the stronger binding ligands such as EDTA, EDTMP, and DTPA had a growth inhibition effect on the microalgae, which is likely due to the competitive behavior of complexation of Fe(III)L in the medium and Fe uptake of phytoplankton. The most essential micronutrients such as Se, Co, Zn, Mn, Cu, and Mo were remained > 50% of initial concentrations in the test medium. In the presence of biodegradable ligands, dissolved Fe(III) species in the growth medium is likely to be precipitated on the algal cells as colloidal form of Fe(III)′ that facilitate Fe uptake into the cells.
References
Andersen RA (2004) Biology and systematics of heterokont and haptophyte algae. Am J Bot 91:1508–1522
Anderson MA, Morel FMM (1982) The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol Oceanogr 27:789–813
Baker JW, Grover JP, Brooks BW, Ureña-Boeck F, Roelke DL, Errera R, Kiesling RL (2007) Growth and toxicity of Prymnesium parvum (Haptophyta) as a function of salinity, light, and temperature. J Phycol 43:219–227
Baker JW, Grover JP, Ramachandrannair R, Black C, Valenti TW Jr, Brooks BW, Roelke DL (2009) Growth at the edge of the niche: an experimental study of the harmful alga Prymnesium parvum. Limnol Oceanogr 54:1679–1687
Beardall J, Berman T, Heraud P, Kadiri MO, Light BR, Patterson G, Roberts S, Sulzberger B, Sahan E, Uehlinger U (2001) A comparison of methods for detection of phosphate limitation in microalgae. Aquat Sci 63:107–121
Begum ZA, Rahman IMM, Tate Y, Egawa Y, Maki T, Hasegawa H (2012) Formation and stability of binary complexes of divalent ecotoxic ions (Ni, Cu, Zn, Cd, Pb) with biodegradable aminopolycarboxylate chelants (DL-2-(2-carboxymethyl) nitrilotriacetic acid, GLDA, and 3-hydroxy-2, 2′-iminodisuccinic acid, HIDS) in aqueous solutions. J Solut Chem 41:1713–1728
Benemann JR (2008) Opportunities and challenges in algae biofuels production. http://www.fao.org/uploads/media/algae_positionpaper.pdf
Benvenuti G, Bosma R, Ji F, Lamers P, Barbosa MJ, Wijffels RH (2016) Batch and semi-continuous microalgal TAG production in lab-scale and outdoor photobioreactors. J Appl Phycol 28:3167–3177
Berne RM, Levy MN (eds) (1998) Physiology. Mosby, USA
Blain S, Quéguiner B, Armand L, Belviso S, Bombled B, Bopp L, Bowie A, Brunet C, Brussaard C, Carlotti F (2007) Effect of natural iron fertilization on carbon sequestration in the Southern Ocean. Nature 446:1070–1074
Boyd P, Berges JA, Harrison PJ (1998) In vitro iron enrichment experiments at iron-rich and-poor sites in the NE subarctic Pacific. J Exp Mar Biol Ecol 227:133–151
Bucheli-Witschel M, Egli T (2001) Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol Rev 25:69–106
Carvalho WF, Granéli E (2010) Contribution of phagotrophy versus autotrophy to Prymnesium parvum growth under nitrogen and phosphorus sufficiency and deficiency. Harmful Algae 9:105–115
Chang FH, Anderson C, Boustead NC (1990) First record of a Heterosigma (Raphidophyceae) bloom with associated mortality of cage-reared salmon in Big Glory Bay, New Zealand. N Z J Mar Freshw Res 24:461–469
Chang FH, Pridmore R, Boustead N (1993) Occurrence and distribution of Heterosigma cf. akashiwo (Raphidophyceae) in a 1989 bloom in Big Glory Bay, New Zealand. In: Smayda TJ, Shimizu Y (eds) Toxic phytoplankton blooms in the sea. Elsevier, Amsterdam, pp 675–680
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Coale KH, Fitzwater SE, Gordon RM, Johnson KS, Barber RT (1996a) Control of community growth and export production by upwelled iron in the equatorial Pacific Ocean. Nature 379:621–624
Coale KH, Johnson KS, Fitzwater SE, Gordon RM, Tanner S, Chavez FP, Ferioli L, Nightingale P, Cooper D, Cochlan WP (1996b) A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 383:495–501
Edler L, Hällfors G, Ak N (1984) Preliminary check-list of the phytoplankton of the Baltic Sea. Acta Bot Fenn 128:1–26
Edvardsen B, Imai I (2006) The ecology of harmful flagellates within Prymnesiophyceae and Raphidophyceae. In: Granéli E, Turner JT (eds) Ecology of harmful algae. Springer, Berlin, pp 67–79
Fistarol GO, Legrand C, Granéli E (2003) Allelopathic effect of Prymnesium parvum on a natural plankton community. Mar Ecol Prog Ser 255:115–125
Gerringa LJA, De Baar HJW, Timmermans KR (2000) A comparison of iron limitation of phytoplankton in natural oceanic waters and laboratory media conditioned with EDTA. Mar Chem 68:335–346
Hasegawa H, Rahman MA, Saitoh K, Ueda K (2010) Effect of biodegradable chelating ligand on iron bioavailability and radish growth. J Plant Nutr 33:933–942
Hasegawa H, Rahman MA, Saitou K, Kobayashi M, Okumura C (2011) Influence of chelating ligands on bioavailability and mobility of iron in plant growth media and their effect on radish growth. Environ Exp Bot 71:345–351
Hasegawa H, Rahman MM, Kadohashi K, Takasugi Y, Tate Y, Maki T, Rahman MA (2012) Significance of the concentration of chelating ligands on Fe3+-solubility, bioavailability, and uptake in rice plant. Plant Physiol Biochem 58:205–211
Hassler CS, Norman L, Mancuso Nichols CA, Clementson LA, Robinson C, Schoemann V, Watson RJ, Doblin MA (2015) Iron associated with exopolymeric substances is highly bioavailable to oceanic phytoplankton. Mar Chem 173:136–147
Hassler CS, Schoemann V, Nichols CM, Butler ECV, Boyd PW (2011) Saccharides enhance iron bioavailability to Southern Ocean phytoplankton. Proc Natl Acad Sci 108:1076–1081
Hudson RJM, Morel FMM (1989) Distinguishing between extra-and intracellular iron in marine phytoplankton. Limnol Oceanogr 34:1113–1120
Hudson RJM, Morel FMM (1990) Iron transport in marine phytoplankton: kinetics of cellular and medium coordination reactions. Limnol Oceanogr 35:1002–1020
Hutchins DA, Franck VM, Brzezinski MA, Bruland KW (1999) Inducing phytoplankton iron limitation in iron-replete coastal waters with a strong chelating ligand. Limnol Oceanogr 44:1009–1018
Hutner SH, Provasoli L, Schatz A, Haskins CP (1950) Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc Am Philos Soc 94:152–170
Hyvönen H, Orama M, Saarinen H, Aksela R (2003) Studies on biodegradable chelating ligands: complexation of iminodisuccinic acid (ISA) with Cu(II), Zn(II), Mn(II) and Fe(III) ions in aqueous solution. Green Chem 5:410–414
Itakura S, Imai I, Itoh K (1992) Morphology and rejuvenation of Skeletonema costatum (Bacillariophyceae) resting cells from the bottom sediments of Hiroshima Bay, the Seto Inland Sea, Japan. Bull Plankton Soc Japan 38:135–145
Itakura S, Imai I, Itoh K (1997) “Seed bank” of coastal planktonic diatoms in bottom sediments of Hiroshima Bay, Seto Inland Sea, Japan. Mar Biol 128:497–508
Jackson GA, Morgan JJ (1978) Trace metal-chelator interactions and phytoplankton growth in seawater media: theoretical analysis and comparison with reported observations. Limnol Oceanogr 23:268–282
Kean MA, Delgado EB, Mensink BP, Bugter MHJ (2015) Iron chelating agents and their effects on the growth of Pseudokirchneriella subcapitata, Chlorella vulgaris, Phaeodactylum tricornutum and Spirulina platensis in comparison to Fe-EDTA. J Algal Biomass Utiln 6:56–73
Knepper TP (2003) Synthetic chelating agents and compounds exhibiting complexing properties in the aquatic environment. Trends Anal Chem 22:708–724
La Claire JW (2006) Analysis of expressed sequence tags from the harmful alga, Prymnesium parvum (Prymnesiophyceae, Haptophyta). Mar Biotechnol 8:534–546
Lin L, Cunshan Z, Vittayapadung S, Xiangqian S, Mingdong D (2011) Opportunities and challenges for biodiesel fuel. Appl Energy 88:1020–1031
Liu Z-Y, Wang G-C, Zhou B-C (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Lundholm N, Moestrup O (2006) The biogeography of harmful algae. In: Granéli E, Turner JT (eds) Ecology of harmful algae. Springer, Berlin, pp 23–35
Lyman J, Fleming RH (1940) Composition of sea water. J Mar Res 3:134–146
Maki T, Suzuki T, Kido K, Nakahara A, Higashi T, Hasegawa H, Ueda K, Saijoh K (2008) Effect of iron stress on gene expression in harmful microalga Prymnesium Parvum. J Ecotechnol Res 14:13–16
Marchetti A, Maldonado MT (2016) Iron. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 233–279
Martell AE, Smith RM (2004) NIST standard reference database 46 version 8.0. NIST critically selected stability constants of metal complexes database, 8.0 edn. US Department of Commerce, Institute of Standards and Technology, MD, USA
Martin JH, Gordon RM, Fitzwater SE (1991) The case for iron. Limnol Oceanogr 36:1793–1802
Moore JK, Doney SC, Glover DM, Fung IY (2001) Iron cycling and nutrient-limitation patterns in surface waters of the World Ocean. Deep Sea Res Part II: Topic Stud Oceanogr 49:463–507
Morel FMM, Hering JG (1993) Principles and applications of aquatic chemistry: complexation. Wiley, New York
Muggli DL, Harrison PJ (1996) EDTA suppresses the growth of oceanic phytoplankton from the Northeast Subarctic Pacific. J Exp Mar Biol Ecol 205:221–227
Myers J, Phillips JN Jr, Graham J-R (1951) On the mass culture of algae. Plant Physiol 26:539–548
Naito K, Imai I, Nakahara H (2008) Complexation of iron by microbial siderophores and effects of iron chelates on the growth of marine microalgae causing red tides. Phycol Res 56:58–67
Nörtemann B (2005) Biodegradation of chelating agents: EDTA, DTPA, PDTA, NTA, and EDDS. In: VanBriesen JM (ed) B. N. Biogeochemistry of Chelating Agents, American Chemical Society, Washington DC, pp 150–170
Okumura C, Rahman MA, Takimoto A, Hasegawa H (2013) Effect of nitrate on the determination of iron concentration in phytoplankton culture medium by liquid scintillation counting (LSC) method using 55Fe as radioisotope tracer. J Radioanal Nucl Chem 296:1295–1302
Park JS (1989) Studies on red tide phenomena in Korean coastal waters. In: Okaichi T, Anderson DM, Nemoto T (eds) Red tides. Elsevier, New York, pp 37–40
Pinto ISS, Neto IFF, Soares HMVM (2014) Biodegradable chelating agents for industrial, domestic, and agricultural applications—a review. Environ Sci Pollut Res 21:11893–11906
Rahman MA, Hasegawa H, Kadohashi K, Maki T, Ueda K (2009) Hydroxyiminodisuccinic acid (HIDS): a novel biodegradable chelating ligand for the increase of iron bioavailability and arsenic phytoextraction. Chemosphere 77:207–213
Rahman MA, Rahman MM, Kadohashi K, Maki T, Hasegawa H (2011) Effect of external iron and arsenic species on chelant-enhanced iron bioavailability and arsenic uptake in rice (Oryza sativa L.) Chemosphere 84:439–445
Rahman MM, Rahman MA, Maki T, Nishiuchi T, Asano T, Hasegawa H (2014) A marine phytoplankton (Prymnesium parvum) up-regulates ABC transporters and several other proteins to acclimatize with Fe-limitation. Chemosphere 95:213–219
Roelke DL, Grover JP, Brooks BW, Glass J, Buzan D, Southard GM, Fries L, Gable GM, Schwierzke-Wade L, Byrd M, Nelson J (2010) A decade of fish-killing Prymnesium parvum blooms in Texas: roles of inflow and salinity. J Plankton Res 33:243–253
Sandy M, Butler A (2009) Microbial iron acquisition: marine and terrestrial siderophores. Chem Rev 109:4580–4595
Shaked Y, Kustka AB, Morel FMM (2005) A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol Oceanogr 50:872–882
Shaked Y, Lis H (2012) Disassembling iron availability to phytoplankton. Front Microbiol 3:123
Shikata T, Nagasoe S, Matsubara T, Yoshikawa S, Yamasaki Y, Shimasaki Y, Oshima Y, Jenkinson IR, Honjo T (2008) Factors influencing the initiation of blooms of the raphidophyte Heterosigma akashiwo and the diatom Skeletonema costatum in a port in Japan. Limnol Oceanogr 53:2503–2518
Sunda WG, Huntsman SA (1995) Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar Chem 50:189–206
Sunda WG, Swift DG, Huntsman SA (1991) Low iron requirement for growth in oceanic phytoplankton. Nature 351:55–57
Susan VJ, Theodore WV, Daniel LR, James PG, Bryan WB (2010) Probabilistic ecological hazard assessment of microcystin-LR allelopathy to Prymnesium parvum. J Plankton Res 33:319–332
Sutak R, Botebol H, Blaiseau PL, Leger T, Bouget FY, Camadro JM, Lesuisse E (2012) A comparative study of iron uptake mechanisms in marine microalgae: iron binding at the cell surface is a critical step. Plant Physiol 160:2271–2284
Taylor FJR, Haigh R (1993) The ecology offish-kills blooms of the chloromonad flagellate Heterosigma in the Strait of Georgia and adjacent waters. In: Smayda TJ, Shimizu Y (eds) Toxic phytoplankton blooms in the sea. Elsevier, Amsterdam, pp 705–710
Trick CG, Andersen RJ, Price NM, Gillam A, Harrison PJ (1983) Examination of hydroxamate-siderophore production by neritic eukaryotic marine phytoplankton. Mar Biol 75:9–17
Watanabe MM, Kawachi M, Hiroki M, Kasai F (2000) NIES collection list of strains: microalgae and protozoa. Microbial culture collections, 6th edn. National Institute for Environmental Studies, Tsukuba
Wells ML, Price NM, Bruland KW (1995) Iron chemistry in seawater and its relationship to phytoplankton: a workshop report. Mar Chem 48:157–182
Yamochi S (1989) Mechanisms for outbreak of Heterosigma akashiwo red tide in Osaka Bay, Japan. In: Nemoto T (ed) Okaichi TA, D. M. Red Tides, Elsevier, New York, pp 253–256
Acknowledgements
We would like to thank Dr. Christel Hassler, University of Geneva, Switzerland, for reviewing the manuscript and making a number of helpful suggestions prior to submission.
Funding
This research was partly supported by a Grant-in-Aid for Scientific Research (18510071) from Japan Society for the Promotion of Science (JSPS) and the Steel Industry Foundation for the Advancement of Environmental Protection Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasegawa, H., Nozawa, A., Papry, R.I. et al. Effect of biodegradable chelating ligands on Fe uptake in and growth of marine microalgae. J Appl Phycol 30, 2215–2225 (2018). https://doi.org/10.1007/s10811-018-1462-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1462-x