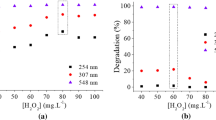
Abstract
The prediction of the system behavior is of significant interest when evaluating appropriate technologies for wastewater treatment. The robust prediction could be achieved by empirical mathematical modeling techniques, but they do not include steps in degradation of organic pollutants. Mechanistic models (MM) include chemical/physical phenomena, but may also include numerous reactions resulting with the complicated kinetic expressions with large number of parameters. This modeling approach can be challenging for complex system such as advanced oxidation processes. With the goal to reduce the number of reactions involved in developed MM, keeping the high prediction power, sensitivity, and flux analyses was employed. The results showed that MM describing the degradation of organic dye in water matrix by photooxidation processes can be significantly simplified, by reducing the number of reactions included without affecting the predictive power. The calculated root mean square deviation values between data predicted by MM and reduced MMR differ insignificantly (≤1.4 %).









Similar content being viewed by others
References
Koprivanac, N., & Kusic, H. (2009). Hazardous organic pollutants in colored wastewaters. New York: Nova Science.
Nilsson, R., Nordlinder, R., & Wass, U. (1993). Asthma, rhinitis, and dermatitis in workers exposed to reactive dyes. British Journal of Industrial Medicine, 50, 65–70.
Ozturk, A., & Abdullah, M. I. (2006). Toxicological effect of indole and its azo dye derivatives on some microorganisms under aerobic conditions. Science of the Total Environment, 358, 137–142.
Forgacs, E., Cserhati, T., & Oros, G. (2004). Removal of synthetic dyes from wastewaters: a review. Environmental International, 30, 953–971.
Gogate, R., & Pandit, B. (2004). A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Advances in Environmental Research, 8, 501–551.
Parsons, S. (2004). Advanced oxidation processes for water and wastewater treatment. London: IWA.
Kamel, D., Sihem, A., Halima, C., & Tahar, S. (2009). Decolourization process of an azoique dye (Congo red) by photochemical methods in homogeneous medium. Desalination, 247, 412–422.
Kusic, H., Jovic, M., Kos, N., Koprivanac, N., & Marin, V. (2010). The comparison of photooxidation processes for the minimization of organic load of colored wastewater applying the response surface methodology. Journal of Hazardous Materials, 183, 189–202.
Peternel, I., Koprivanac, N., & Kusic, H. (2006). UV-based processes for reactive azo dye mineralization. Water Research, 2006, 525–532.
Guimarães, O. L. C., dos Reis Chagas, M. H., Filho, D. N. V., Siqueira, A. F., Filho, H. J. I., de Aquino, H. O. Q., et al. (2008). Discoloration process modeling by neural network. Chemical Engineering Journal, 140, 71–76.
Pontes, R. F. F., Moraes, J. E. F., Machulek, A., Jr., & Pinto, J. M. (2010). A mechanistic kinetic model for phenol degradation by the Fenton process. Journal of Hazardous Materials, 176, 402–413.
Crittenden, J. C., Hu, S., Hand, D. W., & Green, S. A. (1999). A kinetic model for H2O2/UV process in a completely mixed batch reactor. Water Research, 33, 2315–2328.
Kralik, P., Kusic, H., Koprivanac, N., & Loncaric Bozic, A. (2010). Degradation of chlorinated hydrocarbons by UV/H2O2: the application of experimental design and kinetic modeling approach. Chemical Engineering Journal, 158, 154–166.
Lovato, M. E., Martín, C. A., & Cassano, A. E. (2009). A reaction kinetic model for ozone decomposition in aqueous media valid for neutral and acidic pH. Chemical Engineering Journal, 146, 486–497.
Kusic, H., Juretic, D., Koprivanac, N., Marin, V., & Loncaric Bozic, A. (2011). Photooxidation processes for an azo dye in aqueous media: modeling of degradation kinetic and ecological parameters evaluation. Journal of Hazardous Materials, 185, 1558–1568.
Simunovic, M., Kusic, H., Koprivanac, N., & Loncaric Bozic, A. (2011). Treatment of simulated industrial wastewater by photo-Fenton process: Part II. The development of mechanistic model. Chemical Engineering Journal, 173, 280–289.
Grymonpre, D. R., Sharma, A. K., Finney, W. C., & Locke, B. R. (2001). The role of Fenton’s reaction in aqueous phase pulsed streamer corona reactors. Chemical Engineering Journal, 82, 189–207.
Smets, I., Bernaerts, K., Sun, J., Marchal, K., Vanderleyden, J., & Van Impe, J. (2002). Sensitivity function-based model reduction: a bacterial gene expression case study. Biotechnology and Bioengineering, 80, 195–200.
Zhu, J. Y., Dittmeyer, R., & Hofmann, H. (1993). Application of sensitivity analysis to the reduction of a complex kinetic model for the homogeneous oxidative coupling of methane. Chemical Engineering and Processing, 32, 167–176.
Lin, Y.-H., & Wu, C.-L. (2011). Sensitivity analysis of phenol degradation with sulfate reduction under anaerobic conditions. Environmental Modeling and Assessment, 16, 213–225.
Saltelli, A., Ratto, M., Tarantola, S., & Campolongo, F. (2005). Sensitivity analysis for chemical models. Chemical Reviews, 105, 2811–2828.
Kusic, H., Koprivanac, N., Horvat, S., Bakija, S., & Loncaric Bozic, A. (2009). Modeling dye degradation kinetic using dark- and photo-Fenton type processes. Chemical Engineering Journal, 155, 144–154.
Audenaert, W. T. M., Callewaert, M., Nopens, I., Cromphout, J., Vanhoucke, R., Dumoulin, A., et al. (2010). Full-scale modelling of an ozone reactor for drinking water treatment. Chemical Engineering Journal, 157, 551–557.
Liu, G., Swihart, M. T., & Neelamegham, S. (2005). Sensitivity, principal component and flux analysis applied to signal transduction: the case of epidermal growth factor mediated signaling. Bioinformatics, 21, 1194–1202.
Huie, R. E., & Clifton, C. L. (1996). Kinetics of the reaction of the sulfate radical with the oxalate anion. International Journal of Chemical Kinetics, 28, 195–199.
Stylidi, M., Kondarides, D. I., & Verykios, X. E. (2003). Pathways of solar light-induced photocatalytic degradation of azo dyes in aqueous TiO2 suspensions. Applied Catalysis B: Environmental, 40, 271–286.
Connors, K. A. (1990). Chemical kinetics: the study of reaction rates in solution. New York: Wiley-VCH.
Beltran, F. J. (2003). Ozone–UV radiation–hydrogen peroxide oxidation technologies. In M. A. Tarr (Ed.), Chemical degradation methods for wastes and pollutants, environmental and industrial applications (pp. 1–77). New York: Marcel Dekker.
Criquet, J., & Leitner, N. K. V. (2009). Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis. Chemosphere, 77, 194–200.
Liang, C., & Lai, M.-C. (2008). Trichlorethylene degradation by zero valent iron activated persulfate oxidation. Environmental Engineering Science, 25, 1071–1077.
Hoigné, J., & Bader, H. (1983). Rate constants of reactions of ozone with organic and inorganic compounds in water—II: dissociating organic compounds. Water Research, 17, 185–194.
Varma, A., Morbidelli, H., & Wu, H. (1999). Parametric sensitivity in chemical systems. Cambridge: Cambridge University Press.
Buxton, G. V., Greenstock, C. L., Helman, W. P., & Ross, A. B. (1988). Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. Journal of Physical and Chemical Reference Data, 17, 513–586.
Neta, P., Madhavan, V., Zemel, H., & Fesseden, R. W. (1977). Rate constants and mechanism of reaction of SO4•− with aromatic compounds. Journal of American Chemical Society, 99, 163–164.
Lam S.H. (1995). Reduced chemical modeling and sensitivity analysis. Available at www.princeton.edu/∼lam/SHL/VKI.pdf.
Okino, M. S., & Mavrovouniotis, M. L. (1998). Simplification of mathematical models of chemical reaction systems. Chemical Reviews, 98, 391–408.
Bendtsen, A. B., Glarborg, P., & Dam-Johansen, K. (2001). Visualization methods in analysis of detailed chemical kinetic modeling. Computers and Chemistry, 25, 161–170.
Acknowledgments
We would like to acknowledge the financial support from the Ministry of Science, Education and Sport, Republic of Croatia (Project #125-1253092-1981).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 209 kb)
Rights and permissions
About this article
Cite this article
Kusic, H., Koprivanac, N. & Loncaric Bozic, A. Application of Sensitivity and Flux Analyses for the Reduction of Model Predicting the Photooxidative Degradation of an Azo Dye in Aqueous Media. Environ Model Assess 17, 653–671 (2012). https://doi.org/10.1007/s10666-012-9322-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10666-012-9322-6