Abstract
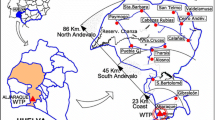
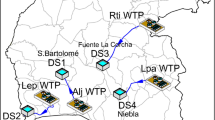
A mathematical model that expresses Total trihalomethane (TTHM) concentration in terms of initial chlorine concentration, total organic carbon, bromide ion concentration, contact time, and pH is developed for Zai water treatment plant which supplies water to Jabal Amman. The developed mathematical model is for constant temperature of 20°C. To adjust model calculated TTHM concentrations for temperatures other than 20°C, another mathematical model that expresses TTHM growth rate as function of temperature is also developed. To test the ability of the two developed models in predicting TTHM concentrations throughout water supplies, a sampling program that aimed at measuring TTHM concentrations in addition to the predictors in the two developed mathematical models namely; chlorine concentration, bromide ion concentration, total organic carbon, temperature and pH throughout Jabal Amman water supply was conducted. The two developed mathematical models and WaterCad, which was used to determine water age, were used to predict TTHM concentrations throughout Jabal Amman water supply. Predicted TTHM concentrations were compared to actual TTHM concentrations measured during the sampling program. Results showed that there is good agreement between measured and, calculated TTHM concentrations, which means that the method presented in this paper, can be used to obtain good estimates of TTHM concentrations throughout networks.
Similar content being viewed by others
References
N. Gary, Drinking Water Quality, Problems and Solutions (Wiley, 1994).
J. Rook (1974) ArticleTitleFormation of haloforms during chlorination of natural waters Water Treat. Examiners 23 IssueID(2) 234–243
T. Bellar J. Lichtenberg R. Kroner (1974) ArticleTitleThe occurrence of organohalides in chlorinated waters J. AWWA 66 IssueID(12) 703–706 Occurrence Handle1:CAS:528:DyaE2MXhtFGqt7s%3D
J. Symons T. Bellar J. Carswell J. DeMarco K. Kropp G. Robeck D. Seeger C. Slocum B. Smith A. Stevens (1975) ArticleTitleNational organic reconnaissance survey for halogenated organics J. AWWA 67 IssueID(11) 634–648 Occurrence Handle1:CAS:528:DyaE28Xks1yjtL8%3D
G. White, Handbook of Chlorination and Alternative Disinfectants (Wiley, 1999).
R. Clark H. Pourmoghaddas L. Wymer R. Dressman (1996) ArticleTitleModeling the kinetics of chlorination by-products formation: The effects of bromide J. SRT-aqua 45 IssueID(3) 112–119 Occurrence Handle1:CAS:528:DyaK28XkslGrsb4%3D
InstitutionalAuthorNameWorld Health Organization (1984) Guidelines for Drinking Water Quality, Vol. 2: Health Criteria and Other Supporting Information World Health Organization Geneva
W. Elshorbagy (2000) ArticleTitleKinetics of THM species in finished water J. Water Resources Planning and Management 126 IssueID(1) 21–28
W. Cooper L. Meyer C. Bofill E. Cordal (1978) ArticleTitleQuantitative effects of bromine on the formation and distribution of trihalomethanes in ground water with a high organic content Water Chlorination: Environmental Impact and Health Effects 3 285–296
R. Minear J. Bird (1978) ArticleTitleTrihalomethanes: impacts of bromide ion concentration on yield, species distribution, rate of formation, and influence of other variables Water Chlorination: Environmental Impact and Other Health Effects 3 151–160
G. Oliver (1978) ArticleTitleEffect of temperature, pH, and bromide concentration on the trihalomethane reaction of chlorine with aquatic humic materials Water Chlorination: Environmental Impact and Health Effects 3 141–149
M. Kavanaugh A. Trussel J. Cormer R. Trussel (1980) ArticleTitleAn empirical kinetic model of trihalomethane formation: Application to meet the proposed THM standard J. AWWA 72 IssueID(10) 578–582 Occurrence Handle1:CAS:528:DyaL3MXlt1KjsA%3D%3D
B. Engerholm G. Amy (1983) ArticleTitlePredictive model for chloroform formation from humic acids J. AWWA 75 IssueID(8) 418–423 Occurrence Handle1:CAS:528:DyaL3sXlt1Oqu7Y%3D
B. Engerholm G. Amy (1983) ArticleTitleAn empirical model for predicting chloroform formation from humic and fulvic acid Water Chlorination: Environmental Impact and Health Effects 3 243–267
C. Haas S. Kara (1984) ArticleTitleKinetics of wastewater chlorine demand exertion J. Water Pollution Control Federation 56 IssueID(2) 120–173
Montgomery Watson Consulting Engineering, Final report reported for AWWA: Mathematical modeling of the formation of THMs and HAAs in chlorinated natural waters, Denver, Colorado (1993).
R. Clark (1998) ArticleTitleChlorine demand and TTHM formation kinetics: a secondorder model J. of Environmental Engineering, ASCE 124 IssueID(1) 16–24 Occurrence Handle1:CAS:528:DyaK1cXjtFai
R. Clark M. Sivaganesan (1998) ArticleTitlePredicting chlorine residuals and formation of TTHMs in drinking water J. of Environmental Engineering, ASCE 124 IssueID(12) 1203–1210 Occurrence Handle1:CAS:528:DyaK1cXns1amt78%3D
R. Clark R. Thurnau M. Sivaganesan P. Ringhand (2001) ArticleTitlePredicting the formation of chlorinated and brominated by-products J. of Environmental Engineering, ASCE 127 IssueID(6) 493–501 Occurrence Handle1:CAS:528:DC%2BD3MXktVWkur8%3D
J. Gould L. Fitchhorn E. Urheim (1981) ArticleTitleFormation of brominated trihalomethanes: extent and kinetics Water Chlorination: Environmental Impacts and Health Effects 4 297–310
W. Bunn B. Hass E. Deane R. Kleopfer (1975) ArticleTitleFormation of trihalomethanes by chlorination of surface waters Environmental Letters 10 IssueID(3) 205 Occurrence Handle10.1080/00139307509435822 Occurrence Handle1:CAS:528:DyaE28Xls1KnsrY%3D
R. Trussel M. Umphres (1978) ArticleTitleThe formation of trihalomethanes J. AWWA 70 IssueID(11) 604–612
H. Pourmoghaddas A. Stevens R. Kinmen R. Dressman L. Moore J. Ireland (1993) ArticleTitleEffect of bromide ion on the formation of HAAs during chlorination J. AWWA 85 IssueID(1) 82–87 Occurrence Handle1:CAS:528:DyaK3sXhsFCjsLs%3D
P. Hutton F. Chung (1994) ArticleTitleBromine distribution factors in THM formation J. Water Resources Planning and Management 120 IssueID(1) 1–16
F. Pontius (1991) ArticleTitleDisinfectant-disinfection-product rule update J. AWWA 83 IssueID(12) 24–115 Occurrence Handle1:CAS:528:DyaK38XptVOgsg%3D%3D
W. Walker (1983) ArticleTitleSignificance of eutrophication in water reservoirs J. AWWA 75 IssueID(1) 38 Occurrence Handle1:CAS:528:DyaL3sXnslOjsQ%3D%3D
N. Palmstorm, R. Carlson and G. Cook, Potential links between eutrophication and the formation of carcinogens in drinking water, Lake and Reservoir Management 4(21) (1988).
A. Karimi P. Singer (1991) ArticleTitleTrihalomethane formation in open reservoirs J. AWWA 83 IssueID(3) 84–88 Occurrence Handle1:CAS:528:DyaK3MXksFKlt7c%3D
A. Martin G. Cooke R. Carlson (1993) ArticleTitleLake sediments as a potential source of trihalomethanes precursors J. Water Res., Oxford, England 27 IssueID(12) 1725–1729 Occurrence Handle1:CAS:528:DyaK3sXmsFKhs7c%3D
R. Canale C. Chapara G. Amy M. Edwards (1997) ArticleTitleTrihalomethane precursor model for Lake Youngs Washington Journal of Environmental Engineering, ASCE 123 IssueID(5) 259–265
P. Hutton F. Chung (1992) ArticleTitleSimulating THM formation potential in Sacramento delta, Part 1 Jouranl of Water Resources Planning and Management 118 IssueID(5) 513–529
Haestad Methods WaterCad v4.1 User’s Guide, Haestad Methods inc. (2000).
A. Al-Nimer, Chlorine decay rate and chlorination efficiency studies for drinking water in Jordan, M.Sc. Thesis, Department of Chemistry, University of Jordan (2002).
Standard Methods for the Examination of Water and Wastewater, 19th edn (American Public Health Association, 1995).
Minitab user’s guide release 13, by Minitab Inc. (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Al-Omari, A., Fayyad, M. & Qader, A.A. Modeling trihalomethane formation for Jabal Amman water supply in Jordan. Environ Model Assess 9, 245–252 (2005). https://doi.org/10.1007/s10666-005-3334-4
Issue Date:
DOI: https://doi.org/10.1007/s10666-005-3334-4