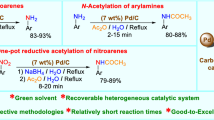
A new method for reduction of N-nitroso aza-aliphatic cyclic compounds employing zinc in pressurized CO2–H2O medium has been developed. H2O and NH4Cl were used as hydrogen donors, and reduction was performed under environmentally benign conditions. The presented approach allowed to obtain the respective N-amino products selectively and in excellent yields (up to 97%).
Similar content being viewed by others
References
(a) Fang, Z.; Yang, Z.; Xu, J.-F.; Guo, K.; Wei, P. Org. Prep. Proced. Int. 2012, 44, 164. (b) Xia, T.; Hu, Z.; Ji, W.; Zhang, S.; Shi, H.; Liu, C.; Pang, B.; Liu, G.; Liao, X. Org. Chem. Front. 2018, 5, 850. (c) Eissa, S. I. Med. Chem. Res. 2017, 26, 2205.
(a) Lucarini, M.; Pedulli, G. F.; Lazzari, D. J. Org. Chem. 2000, 65, 2723. (b) Dhainaut, A.; Régnier, G.; Atassi, G.; Pierré, A.; Léonce, S.; Kraus-Berthier, L.; Prost, J.-F. J. Med. Chem. 1992, 35, 2481.
(a) Zhang, Y.; Tang, Q.; Luo, M. Org. Biomol. Chem. 2011, 9, 4977. (b) Lunn, G.; Sansone, E. B.; Keefer, L. K. J. Org. Chem. 1984, 49, 3470.
(a) Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Org. Process Res. Dev. 2018, 22, 430. (b) Wang, D.; Astruc, D. Chem. Rev. 2015, 115, 6621.
(a) Klager, K.; Wilson, E. M.; Helm-kamp, G. Ind. Eng. Chem. 1960, 52, 119. (b) Tungler, A.; Szabados, E. Org. Process Res. Dev. 2016, 20, 1246.
(a) Kelley, J. L.; Thompson, J. B.; Styles, V. L.; Soroko, F. E.; Cooper, B. R. J. Heterocycl. Chem. 1995, 32, 1423. (b) Bellasio, E.; Campi, A.; Di Mola, N.; Baldoli, E. J. Med. Chem. 1984, 27, 1077.
Manetti, D.; Di Cesare Mannelli, L.; Dei, S.; Galeotti, N.; Ghelardini, C.; Romanelli, M. N.; Scapecchi, S.; Teodori, E.; Pacini, A.; Bartolini, A.; Gualtieri, F. J. Med. Chem. 2005, 48, 6491.
(a) Waibel, M.; Hasserodt, J. J. Org. Chem. 2008, 73, 6119. (b) Klein, J. T.; Davis, L.; Effland, R. C. J. Heterocycl. Chem. 1987, 24, 725.
(a) Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301. (b) Sheldon, R. A. Chem. Soc. Rev. 2012, 41, 1437. (c) Simon, M.-O.; Li, C.-J. Chem. Soc. Rev. 2012, 41, 1415.
(a) Pigaleva, M. A.; Elmanovich, I. V.; Kononevich, Y. N.; Gallyamov, M. O.; Muzafarov, A. M. RSC Adv. 2015, 5, 103573. (b) Jiang, H.-F.; Huang, X.-Z. J. Supercrit. Fluids 2007, 43, 291. (c) Tundo, P.; Loris, A.; Selva, M. Green Chem. 2007, 9, 777. (d) Li, G.; Jiang H.; Li, J. Green Chem. 2001, 3, 250. (e) Gao, G.; Tao, Y.; Jiang, J. Green Chem. 2008, 10, 439. (f) Liu, S.; Wang, Y.; Yang, X.; Jiang J. Res. Chem. Intermed. 2012, 38, 2471. g Jiang, H.-F.; Dong, Y.-S. Chin. J. Chem. 2008, 26, 1407.
(a) Gao, G.; Jiang, J.-Y. Comput. Appl. Chem. 2011, 28, 359 (In Chinese). (b) Roosen, C.; Ansorge-Schumacher, M.; Mang, T.; Leitner, W.; Greiner, L. Green Chem. 2007, 9, 455.
Liu, S.; Wang, Y.; Jiang, J.; Jina, Z. Green Chem. 2009, 11, 1397.
Toews, K. L.; Shroll, R. M.; Wai, C. M.; Smart, N. G. Anal. Chem. 1995, 67, 4040.
(a) Dutcher, B.; Fan, M.; Russell, A. G. ACS Appl. Mater. Interfaces 2015, 7, 2137. (b) Lee, B.; Stowe, H. M.; Lee, K. H.; Hur, N. H.; Hwang, S-J.; Paek, E.; Hwang, G. S. Phys. Chem. Chem. Phys. 2017, 19, 24067.
Kushakova, P. M.; Kuznetsov, V. A.; Chernobroviy, A. N.; Garabadgiu, A. V. Chem. Heterocycl. Compd. 2004, 40, 960. [Khim. Geterotsikl. Soedin. 2004, 1111.]
Mayer, N; Schweiger, M; Melcher, M.-C.; Fledelius, C.; Zechner, R.; Zimmermann, R.; Breinbauer, R. Bioorg. Med. Chem. 2015, 23, 2904.
Ilisson, M.; Tomson, K.; Selyutina, A.; Türk, S.; Mäeorg, U. Synth. Commun. 2015, 45, 1367.
Lebrun, S; Couture, A; Deniau, E; Grandclaudon, P. Synthesis 2006, 3490.
Shaaban, S.; Oh, J.; Maulide, N. Org. Lett. 2016, 18, 345.
Zhou, S.; Wang, J.; Zhang, F.; Song, C.; Zhu, J. Org. Lett. 2016, 18, 2427.
The authors are grateful to the Ministry of Education, ''Chunhui Projects'' project Z2016163) and the Scientific Research Foundation of the Education Department of Sichuan Province (project 12ZB128) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(8), 780–783
Electronic supplementary material
ESM 1
(PDF 2755 kb)
Rights and permissions
About this article
Cite this article
Yang, W., Lu, X., Zhou, T. et al. Selective reduction of N-nitroso aza-aliphatic cyclic compounds to the corresponding N-amino products using zinc dust in CO2–H2O medium. Chem Heterocycl Comp 54, 780–783 (2018). https://doi.org/10.1007/s10593-018-2349-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2349-0