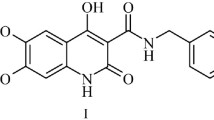
In continuation of the search for potential analgesics amongst 4-hydroxyquinol-2-one derivatives we have proposed and carried out a preparative method of synthesis of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid alkylamides. It has been shown that bromination of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid allylamide using an equivalent of molecular bromine occurs with a conventional addition of the halogen to the allyl double bond and not with halocyclization. The results of the study of the analgesic properties of the compounds prepared are presented.
Similar content being viewed by others
References
V. Ukrainets, N. Yu. Golik, A. L. Shemchuk, and V. N. Kravchenko, Khim. Geterotsikl. Soedin., 1364 (2011). [Chem. Heterocycl. Compd., 47, 1122 (2011)].
Rundshagen, Anästhesiol. Intensivmed. Notfallmed. Schmerzther, 45, 304 (2010).
D. Rainsford, W. F. Kean, and G. E. Ehrlich, Curr. Med. Res. Opin., 24, 2967 (2008).
G. McCleane, Anesthesiol. Clin., 25, 825 (2007).
A. Kleeman and J. Engel, Pharmaceutical Substances. Synthesis, Patents, and Applications, Georg Thieme Verlag, Stuttgart (2001).
M. D. Mashkovskii, Drugs [in Russian], RIA Novaya Volna, Moscow (2009), p. 143.
R. S. Sinatra, J. S. Jahr, and J. M. Watkins-Pitchford (editors), The Essence of Analgesia and Analgesics, Cambridge University Press, Cambridge (2010).
V. Ukrainets, E. V. Mospanova, A. A. Davidenko, and S. V. Shishkina, Khim. Geterotsikl. Soedin., 1345 (2010). [Chem. Heterocycl. Compd., 46, 1084 (2010)].
S. Jönsson, G. Andersson, T. Fex, T. Fristedt, G. Hedlund, K. Jansson, L. Abramo, I. Fritzson, O. Pekarski, A. Runström, H. Sandin, I. Thuvesson, and A. Björk, J. Med. Chem., 47, 2075 (2004).
V. Ukrainets, L. V. Sidorenko, E. N. Svechnikova, and O. V. Shishkin, Khim. Geterotsikl. Soedin., 1503 (2007). [Chem. Heterocycl. Compd., 43, 1275 (2007)].
V. Ukrainets, L. V. Sidorenko, O. V. Gorokhova, S. V. Shishkina, and A. V. Turov, Khim. Geterotsikl. Soedin., 736 (2007). [Chem. Heterocycl. Compd., 43, 617 (2007)].
V. Ukrainets, N. L. Bereznyakova, O. V. Gorokhova, and A. V. Turov, Khim. Geterotsikl. Soedin., 1677 (2007). [Chem. Heterocycl. Compd., 43, 1426 (2007)].
M. A. Mokhort, L. V. Yakovleva, and O. M. Shapoval in: O. V. Stefanov (editor), Preclinical Investigation of Medicinal Agents: Methodological Recommendation [in Ukrainian], Avitsena, Kyiv (2001), p. 307.
N. Sernov and V. V. Gatsura, Elements of Experimental Pharmacology [in Russian], PPP Tipografiya "Nauka", Moscow (2000), p. 40.
V. Ukrainets, E. V. Mospanova, L. V. Savchenkova, and S. I. Yankovich, Khim. Geterotsikl. Soedin., 90 (2011). [Chem. Heterocycl. Compd., 47, 67 (2011).
Ya. A. Sigidin, G. Ya. Shvarts, A. P. Arzamastsev, and S. S. Liberman, Drug Therapy of the Anti- inflammatory Process (Experimental and Clinical Pharmacology of Anti-inflammatory Medications), Meditsina, Moscow (1988), p. 62.
V. Ukrainets, A. A. Davidenko, E. V. Mospanova, L. V. Sidorenko, and E. N. Svechnikova, Khim. Geterotsikl. Soedin., 706 (2010). [Chem. Heterocycl. Compd., 46, 559 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
*For Communication 201, see [1].
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 339-346, February, 2012.
Rights and permissions
About this article
Cite this article
Ukrainets, I.V., Bevz, O.V., Mospanova, E.V. et al. 4-hydroxy-2-quinolones. 202*. Synthesis, chemical and biological properties of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid alkylamides. Chem Heterocycl Comp 48, 320–326 (2012). https://doi.org/10.1007/s10593-012-0992-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0992-4